CHAPTER 6: THERMAL ENERGY
advertisement

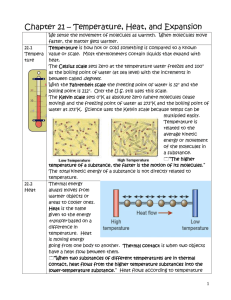
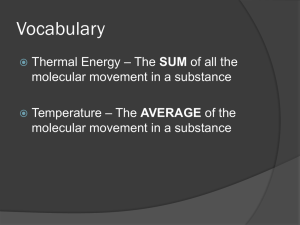
Section 1—TEMPERATURE AND HEAT The temperature of an object is related to the average kinetic energy of the atoms or molecules. These particles move quickly when hot These particles move slowly when cold ↑ KE = ↑ TEMPERATURE The SI unit for temperature is the Kelvin (K) Kinetic energy is energy in the form of motion. KE depends on the mass and the velocity of an object. MASS—how much matter is in an object VELOCITY—speed and direction of an object All matter is made of tiny particles—atoms and molecules. Atoms make up molecules and are held together by chemical bonds. In all materials---solids, liquids, or gases, these particles are in constant motion. The sum of the kinetic and potential energy of all molecules in an object is the thermal energy of the object. ↑ SPEED of the molecules = ↑ KE ↑ DISTANCE (separation) = ↑ PE Heat always flows from warmer to cooler objects. Examples— A cup of hot chocolate/your cold hands Warm air/cold stick of butter Because the air in the room is a higher temperature than the butter, molecules in the air have more KE than the butter molecules. Energy is transferred from faster-moving molecules in the air to slower-moving butter molecules. The butter molecules then begin to move faster and the temperature of the butter increases. ↑ temperature = ↑ thermal energy (KE) (KE + PE) HEAT is THERMAL ENERGY that flows from something at a higher temperature to something at a lower temperature. Example—CHAIR Thermal energy from a person’s body flowed to the chair and increased the temperature of the chair. Heat is a form of energy, so it is measured in joules—the same unit that energy is measured in . Heat always flows from warmer to cooler materials. The amount of heat that is needed to raise the temperature of 1 kg of some material by 1°C or 1 K is called the specific heat of the material. Specific heat is measured in joules per kilogram Kelvin or J/kgK. Compared to 1 kg of sand, the amount of heat that is needed to raise the temperature of 1 kg of water by 1 °C is about 6 times greater. So…the ocean water at the beach would have to absorb 6 times as much heat as the sand to be at the same temperature. Because water can absorb heat without a large change in temperature, it is useful as a coolant. A coolant is a substance that is used to absorb heat. Section 2—Transferring Thermal Energy Thermal energy travels as heat from a material at a higher temperature to a material at a lower temperature. The transfer of thermal energy from matter by the direct contact of particles is called CONDUCTION. Examples—making a snowball; drinking a cup of hot chocolate (DIRECT CONTACT) ***CONDUCTION occurs because all matter is made up of atoms and molecules that are in constant motion. Heat can be transferred by conduction from one material to another (SOUP TO SPOON) or through one material (FROM ONE END OF THE SPOON TO THE OTHER END). Although, CONDUCTION can occur in solids, liquids, and gases---solids usually conduct heat much more effectively Why do you think most cooking pots are made of metal, but the handles usually are not? Silver, copper, and aluminum are among the best heat conductors. Wood, plastic, glass, and fiberglass are poor conductors of heat. One way liquids and gases differ from solids is that they can flow. Any material that can flow allows fluids to transfer heat in another way—CONVECTION. CONVECTION is the transfer of energy in a fluid by the movement of the heated particles. Example—Earth’s atmosphere is made of various gases and is a fluid. The atmosphere is warmer at the equator than at the poles. Also, the atmosphere is warmer at Earth’s surface than higher altitudes. These temperature differences create convection currents that carry heat to cooler regions. Earth gets heat from the Sun, but how does that heat travel through space? ***Almost no matter exists in the space between Earth and the Sun, so heat cannot be transferred by CONDUCTION OR CONVECTION. Instead, the Sun’s heat reaches the Earth by RADIATION. Energy that is transferred by radiation is often called RADIANT ENERGY. Light-colored clothing---reflects more radiant energy Dark-colored clothing---absorbs more radiant energy A material that does not allow heat to flow through it easily is called an INSULATOR. Materials such as wood, plastic, and fiberglass are good insulators and therefore, are poor conductors of heat. Gases, such as air, are usually better insulators than solids or liquids. A material that is a good conductor of heat, such as a metal, is a poor insulator. Air = good insulator, poor conductor Building insulation is usually made of fluffy material, such as fiberglass, that contains pockets of trapped air. Reduces flow of heat between building and the air outside Helps furnaces and air conditioners work more effectively, saving energy Section 3—Using Heat What is the simplest and oldest heating system? Wood or coal burned in a stove Advantage—cheap Disadvantage—heat transfer to others rooms is slow Most common--PHS Fuel is burned in a furnace and heats air; fan blows warm air through ducts in each room CONVECTION CURRENT Closed metal container that contains hot water or steam. Fuel is burned in a central furnace and heats a tank of water; pipes carry the hot water to radiators that are located in each room. No central furnace Cost more than electric heat pump Walls/floors not able to include pipes/ducts The energy from the Sun is called solar energy. Advantage—free, endless supply Disadvantage—depends on location/weather No mechanical devices House/building faces the South (lots of windows on the South sunny side) Cheap Uses devices called solar collectors; heat air or water and then circulate it through the house Expensive