gas law review game - Palatine High School
advertisement

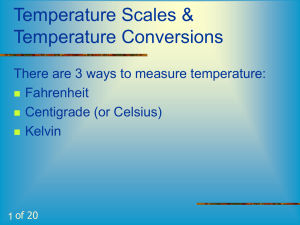
Gas Law Gas Law CalcConcepts ulations STP & Gas Law Temp. Demos Conversions SCUBA & GreenHouse Effect 100 100 100 100 100 200 200 200 200 200 300 300 300 300 300 400 400 400 400 400 500 500 500 500 500 Final Jeopardy Question Experiment: Student A begins boiling a cup of water in Boston while Student B does the same at the same time in Denver. Which student’s water will boil first and WHY? Back Student B (in Denver) since the atmospheric pressure is lower in Denver (higher altitude), water here will boil first. The water in Denver will not need to meet as high of an atmospheric pressure as that in Boston. Back As you go down in elevation, what happens to the atmospheric pressure and WHY? Back Pressure increases as elevation decreases because the air is more dense at lower elevations. Back What does John’s Law state and what is the equation? Back As pressure goes up, temperature goes up (and vice-versa) at constant volume. P1 = P2 T1 T2 Back What is the equation for Boyle’s law? Back P1V1 = P2V2 Back What does Charles’ Law state? Back At constant pressure, as temperature increases, volume increases (and viceversa). Back A sample of gas has a volume of 23 mL at 39oC and 890 mm Hg. This sample is cooled down to 11oC and now has a volume of 150 mL. What is the new pressure? Back Use the combined gas law since all variables are changing… P1V1 = T1 P2V2 T2 890 mm Hg ( 23 mL) 312 K P2 = 124.22 mm Hg = P2 (150 mL) 284 K Back A 50 L container is filled with a gas to a pressure of 4.7 atm at 32oC. At what temperature IN DEGREES CELSIUS will the pressure inside the container be 3.5 atm with a volume of 50 L? Back Volume is constant, so use John’s law. P1 = P2 T1 T2 4.7 atm = 3.5 atm 305 K T2 T2 = 227.13 K = -45.87 oC Back A gas occupies a volume of 34 mL at 21.8 oC. To what temperature (in Kelvin) must the gas be raised to have a volume of 86 mL? Assume constant pressure. Back Pressure is constant, so use Charles’ law. V1 = V2 T1 T2 34 mL = 86 mL 294.8 K T2 T2 = 745.67 K Back A balloon has a volume of 6L at 13.2 psi. If the balloon was brought to a pressure of 9.3 psi, what would the new volume be (assume constant temp). Back Temp is constant, so use Boyle’s law. P1V1 = P2V2 13.2 psi (6L) = 9.3 psi (V2) V2 = 8.52 L Back Whose law is being used in the following situation… A sample of gas in a flexible container occupies 46.5 mL at standard pressure. What volume will it take up if the pressure was increased to 3 atm? Assume constant temp. Back Temp is held constant, so it is Boyle’s law. Back Convert 280.1 kPa to mm Hg. Back 760 mm Hg x 280.1 kPa 101.3 kPa = 2101.44 mm Hg Back What is the Kelvin value for absolute zero, and what theoretically happens at this temperature? Back ZERO Kelvin (0K), and everything stops moving at this temp. Back What is 550K in oC? Back 277 oC K = 273 + oC SO… oC = K-273 Back What is 38.5 oC in Kelvin? Back 311.5 K K = 273 + oC Back What are the values for STP in kPa and torr? Back 101 kPa and 760 torr Back Explain how a straw works – and DO NOT USE THE WORD “SUCK”. Back When you inhale, your body takes in the air that is in a straw, creating less pressure in the straw. Since the pressure in the straw is less than the pressure outside the straw, the atmospheric pressure PUSHES the liquid into the straw so you can enjoy! Thank heaven for atmospheric pressure! Back Explain the egg in flask demo. AND DO NOT USE THE PHRASE “SUCKED IN”. Back In the egg demo, water was heated in the flask. Since temp increased in the flask, the pressure also increased. The flask was then taken away from the heat, and an egg was placed on top. The flask cools, so the pressure inside the flask decreased. Since the pressure inside the flask decreased, the atmospheric pressure outside the flask PUSHED the egg into the flask. Back A pressure apparatus that contains a sample of gas at a fixed volume is first placed into a container of hot water. It is then plunged into a container of liquid nitrogen. What happens to the pressure and why? Back This is an example of John’s law – when temp goes down, pressure goes down. Back Explain why the Ivory soap expanded in the microwave. Back Since temp increased, so does volume. This is Charles’ law. Back What will happen to the volume of a balloon if it is placed into a bell jar (vacuum pump) and the pump is turned on. Hint: in a bell jar, the pressure inside decreases. Back The volume of the balloon will increase. This is Boyle’s law… as P decreases, V increases and vice versa. Back What is the relationship between pressure and depth as you descend during a SCUBA dive? Back Every 10 meters (or 32 feet) you descend is an increase in 1 atm. Back What would happen if someone ascended too quickly during a SCUBA dive? Back The lungs of the diver can expand too quickly and tear. Since pressure decreases as you ascend, volume will increase (Boyle’s law). Back What has caused an increase in global warming (greenhouse effect) over the past 100 years? Back Gases in the earth’s atmosphere absorb heat coming from the earth’s surface and reflect it back to the earth. This warms the earth. Through our daily activities such as driving cars, using a BBQ grill, using air conditioners and refrigerators, more gases are emitted into the environment which increases global warming. Back What is the relationship between the solubility of a gas and depth of descent? Back As you descend further (elevation decreases), the solubility of a gas increases. So, at deeper depths, you will have more gas dissolved in your body. Back What is one characteristic of the ozone layer? Back *absorbs UV light *thin layer surrounding the earth *depletion could increase the amount of UV rays hitting the earth which could mean increased mutations. Back Type Question Here Back Type Answer Here Back Type Question Here Back Type Answer Here Back Type question Here Back Type Answer Here Back Type Answer Here Back Type Answer Here Back Type Question Here Back Type Answer Here Back If you are making pasta in Los Angeles and at the same time, your best friend in Colorado Springs is doing the exact same thing, whose pasta will cook first and why? Back LOS ANGELES – the boiling point of water in Los Angeles is higher than the boiling point of water in Colorado Springs. WHY? Water boils when the vapor pressure equals the atmospheric pressure. Because the atmospheric pressure in L.A. is higher than that in Colorado Springs, so even though the water in L.A. will boil second, it will have a higher temperature providing more heat. THUS, the pasta in L.A. will be done first. Back This Jeopardy Game was Created by: Sara Feltman, 5th Grade Teacher Scott Elementary School 1999-2000 School Year Revised: RLipkowitz 2000