Gay Lussac`s Law
advertisement

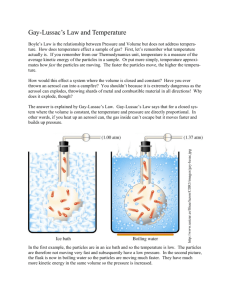
Gay-Lussac's Law Joseph-Louis Gay-Lussac In the early 1800’s, Gay-Lussac ascended to a height of approximately 23,000 ft in a hot air balloon to study variations in the Earth's electro-magnetic intensity relative to altitude. On this flight, he experienced the effects of oxygen deprivation but still managed to collect air samples at over 20,000 ft, study the variation of pressure and temperature, and continue his observations on electromagnetism. What is Gay-Lussac’s Law? Gay-Lussac's Law states that the pressure of a sample of gas at constant volume, is directly proportional to its temperature in Kelvin. Mathematically Gay-Lussac’s Law can be expressed as P1 = P2 T1 T2 P1 T2 = P2 T1 • • • • P1 is the initial pressure T1 is the initial temperature (in Kelvin) P2 is the final pressure T2 is the final temperature (in Kelvin) Gay-Lussacs’s Law Practice Problems 1. A tank of gas has a pressure of 2.75 atm at 20°C. What will be the new pressure in the tank if its temperature is increased to 100°C? P1 T2 = P2 T1 P1: T1: P2: T2: 2. A gas system has initial pressure of 793 kPa and temperature of 34.5°C If the pressure changes to 446 kPa, what will the resultant temperature be in K? P1 T2 = P2 T1 P1: T1: P2: T2: COMBINED GAS LAW The combined gas law is a combination of Boyle's Law and Charles's Law; hence its name the combined gas law. In the combined gas law, the volume of gas is directly proportional to the absolute temperature and inversely proportional to the pressure. Mathematically, Combined Gas Law can be expressed as P1V1 = P2V2 T1 T2 • • • • • • P1V1 T2 =P2V2 T1 P1 is the initial pressure V1 is the initial volume T1 is the initial temperature (in Kelvin) P2 is the final pressure V2 is the final volume T2 is the final temperature (in Kelvin) Combined Gas Law Practice Problems 1. A sample of gas occupies a volume of 75 mL at a pressure of 725 mmHg and a temperature of 18°C. What volume would this sample of gas occupy at 800 mmHg and 298K? P1V1 T2 =P2V2 T1 P1: V1: T1: P2: V2: T: 2. A sample of gas occupies a volume of 3.75 L at a pressure of 1.15 atm and a temperature of 25°C. At what temperature will the sample of gas occupy 4 L at a pressure of 1 atm? P1V1 T2 =P2V2 T1 P1: V1: T1: P2: V2: T2: