ice nucleus
advertisement
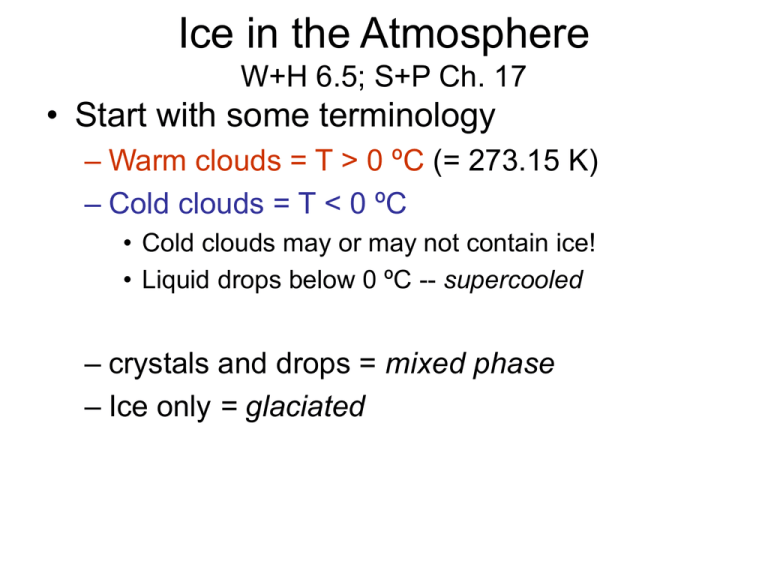
Ice in the Atmosphere W+H 6.5; S+P Ch. 17 • Start with some terminology – Warm clouds = T > 0 ºC (= 273.15 K) – Cold clouds = T < 0 ºC • Cold clouds may or may not contain ice! • Liquid drops below 0 ºC -- supercooled – crystals and drops = mixed phase – Ice only = glaciated Zone of ice clouds (Using global mean profile) z G = 6.5 K/km Cold and glaciated ~8 km -40 ºC Cold zone 2 km 0 ºC Warm zone T 15 ºC Frequency of occurrence How does it get to be this way? • Clouds forming in cold conditions are ice clouds • Clouds forming in warm conditions, then transported to cold conditions can contain supercooled water State of cold clouds vs. temp % Containing some ice Marine (clean) Continental Freezing requires nucleation To create an ice crystal, a surface must be created. This requires a “nucleation” event, just as formation of liquid drops in saturated air requires nucleation. Homogeneous freezing of liquid water occurs at ~-40 ºC If an aerosol in the air or the liquid drop has a crystal matrix similar to ice, it can cause heterogeneous freezing between 0 ºC and -40 ºC. AgI is a classic heterogeneous nucleus Method of Ice Nucleation • Homogeneous Freezing of liquid water drops – No solids in supercooled drop • Heterogeneous Nucleation – Freezing mediated by an ice nucleus (IN) – Types of Heterogeneous Ice Nucleation • Freezing Nucleus – Impurity in a drop • Contact Nucleus – IN in air contacts a drop + starts freezing • Deposition Nucleus – Supersaturation in air w/respect to ice deposits on an aerosol particle Clausius Clapyron & Phase Diagram General Equation: dps (T ) L M W pS dT RT 2 L = LS for sublimation L = LV for vaporization Since LS > LV, supercooled H2O is always supersaturated with respect to ice When ambient vapor pressure is above both curves, vapor condenses to both ice and supercooled drops. When ambient vapor falls between the two curves, drops evaporate, and crystals grow. Note: Some IN work better when liquid deposits first, and then freezes: Parameterization of IN Concentrations For CCN, we used power law with respect to supersaturation S N ( s) C 1% k where C and k are a best fit to observations, or perhaps based on a powerlaw aerosol size distribution For IN, we base things on freezing temperature instead of supersaturation. and for which NIN is measured in particles per liter (i.e. 10-3 cm-3) using: N IN (T ) expaT1 T OR N IN (T ) expaT1 T bsi The warmest freezing temperatures (-4 ºC) tend to be primary organics (leaf debris, plankton, etc). Certain types of mica dust are good in the -10 to -20 ºC range. The Freezing Process • Latent heat is released during droplet freezing. – The droplet is warmer than surroundings during freezing – Pronounced for supercooled drops suddenly freezing. • Lf = 3.3x105 J/kg; • Cpw = 4.2x103 J/kg/K – Lf/Cpw = 78 ºC! • This means a supercooled droplet would have to be -78 ºC at the beginning of the freezing process for it to completely freeze after nucleation. • If surface of drop freezes prior to interior, it may explode (ice multiplication)… freezing is an expansive process – This may explain the observation that observed ice crystal densities far exceed the observed IN concentrations – Another explanation is the potential that H2SO4 crystals may survive convection and act as IN – A third is that fragile ice crystals may break up. Growth of ice crystals • Growth by deposition: – Produces a number of crystalline forms, based on the crystal’s history of temperature and supersaturation. • Plate-like vs. Column-like • http://www.its.caltech.edu/~atomic/snowcrystals/primer/prime r.htm • Growth by riming – Ice crystals collide with supercooled drops when falling, and get coated with rapidly frozen drops. Growth by deposition in mixed phase clouds Consider NC ice crystals of mean diameter DpC, and ND supercooled drops of mean diameter DpD dM M w 2Dv D p (nv neq ) Each grow by diffusion: dt The net impact of deposition to the particles on the ambient vapor concentration is: dnv N C 2Dv D pC (nv neq,ice ) N D 2Dv D pD (nv neq,liq ) dt Suppose now that we assert steady-state conditions – dnv 0 N C 2Dv D pC (nv neq,ice ) N D 2Dv D pD (nv neq,liq ) dt N D DpD (neq,liq neq,ice ) dMC This yields MW 2Dv DpC dt NC DpC N D DpD NC DpC (neq,ice ) N D DpD (neq,liq ) nv NC DpC N D DpD NC DpC (neq,liq neq,ice ) dMD MW 2Dv DpD dt NC DpC N D DpD Growth by deposition – crystal habits • Key controlling factors: T, sliq Guide to snowflakes Kenneth Librecht http://www.its.caltech.edu/~at omic/snowcrystals/class/class .htm








