Socrative Quiz - Spring Lake Park Schools
advertisement

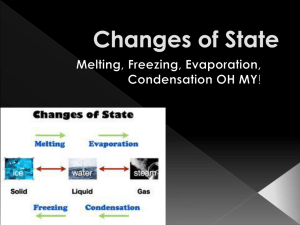
Socrative Quiz - Temperature • Question 1 • Define temperature in your own words. • Temperature is the measurement of the average movement (kinetic energy) of the particles in a substance or object. • Question 2 • Describe thermal expansion in terms of the movement of particles. • When particles get hot, they move faster, gaining kinetic energy, and start to spread out. Therefore the particles take up more space. • Question 3 • Describe thermal expansion in terms of the movement of particles. • When particles get hot, they move faster, gain kinetic energy, and spread out more. Therefore the particles take up more space. • Question 4 • Which is the coldest temperature? • 0 K is the coldest temperature. It is the same as -273 degrees C (not F). • Question 5 • Does temperature depend on the amount of the substance? Explain. • Temperature of a substance only depends on how fast or slow the particles are moving. It does not depend on how many particles there are in the substance. • Question 6 • Why do you think heating a full pot of soup on the stove could cause the soup to overflow? • When particles get hot, they move faster and spread out (expand) so they take up more space. So if the pot is totally full of soup, it will overflow when the particles spread out. • Question 7 • What happens to the density of a substance during thermal expansion? • The amount (mass) of the substance stays the same but since hot particles move faster than cold particles, they will spread out and take up more space. So hot substances are less dense than cold substances. • Question 8 • A glass of cold water whose particles had a low average kinetic energy was placed on a table. The average kinetic energy of the water in the glass increased, while the average kinetic energy of the part of the table under the glass decreased. Explain what you think happened. • The warmer table (more kinetic energy) transferred some of its heat energy through conduction to the colder particles (less kinetic energy) in the glass, thereby making the table colder and the water in the glass warmer. This is because heat moves from warmer areas to colder areas.