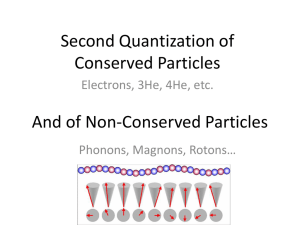
SNC1D Particle Theory
advertisement
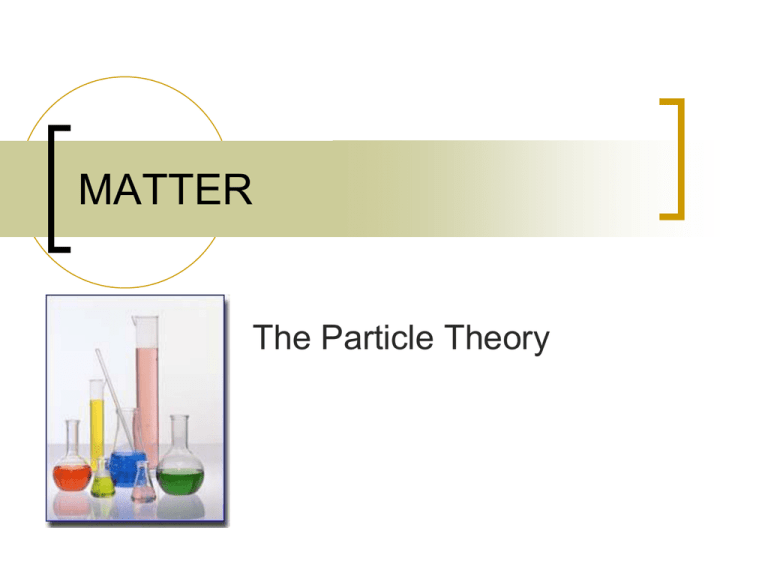
MATTER The Particle Theory Particle Theory of Matter 1. All matter is made of small particles Particle Theory of Matter 2. Each substance has its own kind of particle, which is different from other substances Particle Theory of Matter 3. Particles attract each other • When particles are closer, the attraction is stronger 4. Particles are always moving • • Particles possess kinetic energy Particles vibrate, rotate, and translate Particles attract and always moving Particle Theory of Matter 5. Particles move quickly at high temperatures, and move slowly at low temperatures heating and cooling particles Explain Solids have a hard rigid shape and a definite volume #3 – particles are very close together and attract eachother Explain Carbon monoxide is a poisonous gas but carbon dioxide is not, even though they both consist of carbon and oxygen #2 – each substance has its own kind of particle which is different from other substances Explain A balloon filled with helium gas shrinks when taken outside on a cold winter day #5 – particles move more slowly at cooler temperatures and thus, move closer together Explain In which container will a tablespoon of sugar dissolve faster: in a cup of hot tea or in a glass of iced tea (neither is stirred) #5 – in a cup of hot tea: particles are moving faster and therefore, they spread apart more quickly