Anatomical pathways of the posterior fusiform gyrus
advertisement
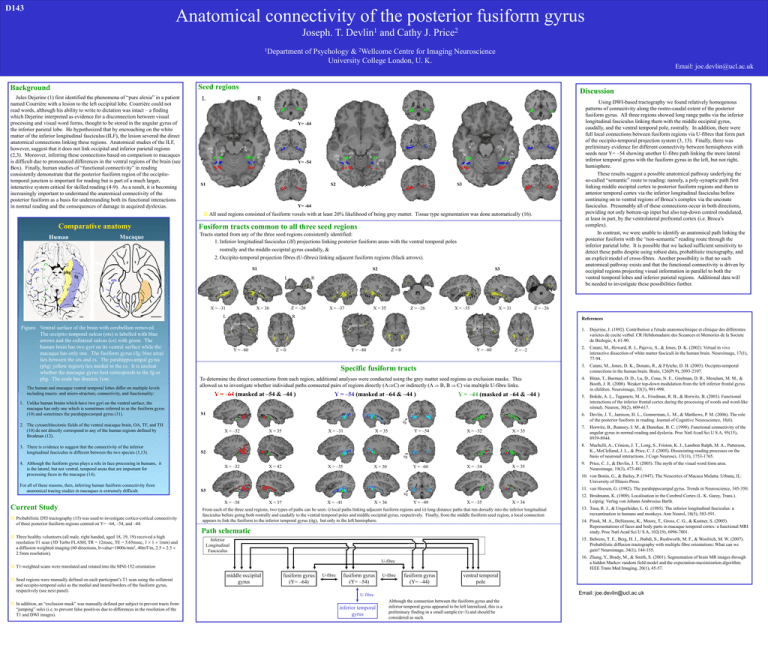
D143 Anatomical connectivity of the posterior fusiform gyrus 1 Devlin Joseph. T. 1Department and Cathy J. 2 Price of Psychology & 2Wellcome Centre for Imaging Neuroscience University College London, U. K. Email: joe.devlin@ucl.ac.uk Seed regions Background Jules Dejerine (1) first identified the phenomena of “pure alexia” in a patient named Courrière with a lesion to the left occipital lobe. Courrière could not read words, although his ability to write to dictation was intact – a finding which Dejerine interpreted as evidence for a disconnection between visual processing and visual word forms, thought to be stored in the angular gyrus of the inferior parietal lobe. He hypothesized that by encroaching on the white matter of the inferior longitudinal fasciculus (ILF), the lesion severed the direct anatomical connections linking these regions. Anatomical studies of the ILF, however, suggest that it does not link occipital and inferior parietal regions (2,3). Moreover, inferring these connections based on comparison to macaques is difficult due to pronounced differences in the ventral regions of the brain (see Box). Finally, human studies of “functional connectivity” in reading consistently demonstrate that the posterior fusiform region of the occipitotemporal junction is important for reading but is part of a much larger, interactive system critical for skilled reading (4-9). As a result, it is becoming increasingly important to understand the anatomical connectivity of the posterior fusiform as a basis for understanding both its functional interactions in normal reading and the consequences of damage in acquired dyslexias. Discussion L R Using DWI-based tractography we found relatively homogenous patterns of connectivity along the rostro-caudal extent of the posterior fusiform gyrus. All three regions showed long range paths via the inferior longitudinal fasciculus linking them with the middle occipital gyrus, caudally, and the ventral temporal pole, rostrally. In addition, there were full local connections between fusiform regions via U-fibres that form part of the occipito-temporal projection system (3, 13). Finally, there was preliminary evidence for different connectivity between hemispheres with seeds near Y= –54 showing another U-fibre path linking the more lateral inferior temporal gyrus with the fusiform gyrus in the left, but not right, hemisphere. These results suggest a possible anatomical pathway underlying the so-called “semantic” route to reading: namely, a poly-synaptic path first linking middle occipital cortex to posterior fusiform regions and then to anterior temporal cortex via the inferior longitudinal fasciculus before continuing on to ventral regions of Broca’s complex via the uncinate fasciculus. Presumably all of these connections occur in both directions, providing not only bottom-up input but also top-down control modulated, at least in part, by the ventrolateral prefrontal cortex (i.e. Broca’s complex). In contrast, we were unable to identify an anatomical path linking the posterior fusiform with the “non-semantic” reading route through the inferior parietal lobe. It is possible that we lacked sufficient sensitivity to detect these paths despite using robust data, probablistic tractography, and an explicit model of cross-fibres. Another possibility is that no such anatomical pathway exists and that the functional connectivity is driven by occipital regions projecting visual information in parallel to both the ventral temporal lobes and inferior parietal regions. Additional data will be needed to investigate these possibilities further. Y= -44 ots cs cs cs ots ots ots cs cs cs ots ots Y= -54 S1 S2 S3 Y= -64 All seed regions consisted of fusiform voxels with at least 20% likelihood of being grey matter. Tissue type segmentation was done automatically (16). Comparative anatomy Macaque Human Fusiform tracts common to all three seed regions Tracts started from any of the three seed regions consistently identified: 1. Inferior longitudinal fasciculus (ilf) projections linking posterior fusiform areas with the ventral temporal poles rostrally and the middle occipital gyrus caudally, & 2. Occipito-temporal projection fibres (U-fibres) linking adjacent fusiform regions (black arrows). cs ots S1 phg fg S2 L ots X = –31 Z = –26 X = 36 L Figure: Ventral surface of the brain with cerebellum removed. The occipito-temporal sulcus (ots) is labelled with blue arrows and the collateral sulcus (cs) with green. The human brain has two gyri on its ventral surface while the macaque has only one. The fusiform gyrus (fg; blue area) lies between the ots and cs. The parahippocampal gyrus (phg; yellow region) lies medial to the cs. It is unclear whether the macaque gyrus best corresponds to the fg or phg. The scale bar denotes 1cm. The human and macaque ventral temporal lobes differ on multiple levels including macro- and micro-structure, connectivity, and functionality: 1. Unlike human brains which have two gyri on the ventral surface, the macaque has only one which is sometimes referred to as the fusiform gyrus (10) and sometimes the parahippocampal gyrus (11). ilf ilf X = –37 X = 35 ilf Z = –26 ilf X = –35 Z = –26 X = 31 R References mid-occ Y = –80 mid-occ mid-occ Y = –80 Z=0 Y = –80 Z=0 Z = –2 Specific fusiform tracts To determine the direct connections from each region, additional analyses were conducted using the grey matter seed regions as exclusion masks. This allowed us to investigate whether individual paths connected pairs of regions directly (AC) or indirectly (A B, B C) via multiple U-fibre links. Y = –64 (masked at –54 & –44 ) Y = –54 (masked at –64 & –44 ) Y = –44 (masked at –64 & –44 ) S1 1. Dejerine, J. (1892). Contribution a l'etude anatomoclinique et clinique des differentes varietes de cecite verbal. CR Hebdomadaire des Sceances et Memories de la Societe de Biologie, 4, 61-90. 2. Catani, M., Howard, R. J., Pajevic, S., & Jones, D. K. (2002). Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage, 17(1), 77-94. 3. Catani, M., Jones, D. K., Donato, R., & Ffytche, D. H. (2003). Occipito-temporal connections in the human brain. Brain, 126(Pt 9), 2093-2107. 4. Bitan, T., Burman, D. D., Lu, D., Cone, N. E., Gitelman, D. R., Mesulam, M. M., & Booth, J. R. (2006). Weaker top-down modulation from the left inferior frontal gyrus in children. Neuroimage, 33(3), 991-998. 5. Bokde, A. L., Tagamets, M. A., Friedman, R. B., & Horwitz, B. (2001). Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron, 30(2), 609-617. 6. Devlin, J. T., Jamison, H. L., Gonnerman, L. M., & Matthews, P. M. (2006). The role of the posterior fusiform in reading. Journal of Cognitive Neuroscience, 18(6). 7. Horwitz, B., Rumsey, J. M., & Donohue, B. C. (1998). Functional connectivity of the angular gyrus in normal reading and dyslexia. Proc Natl Acad Sci U S A, 95(15), 8939-8944. 8. Mechelli, A., Crinion, J. T., Long, S., Friston, K. J., Lambon Ralph, M. A., Patterson, K., McClelland, J. L., & Price, C. J. (2005). Dissociating reading processes on the basis of neuronal interactions. J Cogn Neurosci, 17(11), 1753-1765. 9. Price, C. J., & Devlin, J. T. (2003). The myth of the visual word form area. Neuroimage, 19(3), 473-481. itg 2. The cytoarchitectonic fields of the ventral macaque brain, OA, TF, and TH (10) do not directly correspond to any of the human regions defined by Brodman (12). 3. There is evidence to suggest that the connectivity of the inferior longitudinal fasciculus is different between the two species (3,13). R ilf ilf S3 X = –32 X = –31 X = 35 Y = –54 X = 35 X = –32 X = 35 S2 itg 4. Although the fusiform gyrus plays a role in face processing in humans, it is the lateral, but not ventral, temporal areas that are important for processing faces in the macaque (14). For all of these reasons, then, inferring human fusiform connectivity from anatomical tracing studies in macaques is extremely difficult. Current Study Probabilistic DTI tractography (15) was used to investigate cortico-cortical connectivity of three posterior fusiform regions centred on Y= –64, –54, and –44. Three healthy volunteers (all male, right handed, aged 18, 19, 19) received a high resolution T1 scan (3D Turbo FLASH, TR = 12msec, TE = 5.65msec, 1 1 1mm) and a diffusion weighted imaging (60 directions, b-value=1000s/mm2, 40mT/m, 2.5 2.5 2.5mm resolution). X = –32 X = –35 X = 42 Y = –60 X = 30 X = –34 10. von Bonin, G., & Bailey, P. (1947). The Neocortex of Macaca Mulatta. Urbana, IL: University of Illinois Press. 11. van Hoesen, G. (1982). The parahippocampal gyrus. Trends in Neuroscience, 345-350. S3 itg X = –38 X = –41 X = 37 Y = –49 X = 36 X = –35 Path schematic middle occipital gyrus 12. Brodmann, K. (1909). Localisation in the Cerebral Cortex (L. K. Garey, Trans.). Leipzig: Verlag von Johann Ambrosias Barth. 13. Tusa, R. J., & Ungerleider, L. G. (1985). The inferior longitudinal fasciculus: a reexamination in humans and monkeys. Ann Neurol, 18(5), 583-591. 14. Pinsk, M. A., DeSimone, K., Moore, T., Gross, C. G., & Kastner, S. (2005). Representations of faces and body parts in macaque temporal cortex: a functional MRI study. Proc Natl Acad Sci U S A, 102(19), 6996-7001. 15. Behrens, T. E., Berg, H. J., Jbabdi, S., Rushworth, M. F., & Woolrich, M. W. (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 34(1), 144-155. Inferior Longitudinal Fasciculus 16. Zhang, Y., Brady, M., & Smith, S. (2001). Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging, 20(1), 45-57. T1-weighted scans were translated and rotated into the MNI-152 orientation fusiform gyrus (Y= –64) U-fibre fusiform gyrus (Y= –54) U-fibre fusiform gyrus (Y= –44) ventral temporal pole Email: joe.devlin@ucl.ac.uk U-fibre In addition, an “exclusion mask” was manually defined per subject to prevent tracts from “jumping” sulci (i.e. to prevent false positives due to differences in the resolution of the T1 and DWI images). X = 34 From each of the three seed regions, two types of paths can be seen: i) local paths linking adjacent fusiform regions and ii) long distance paths that run dorsally into the inferior longitudinal fasciculus before going both rostrally and caudally to the ventral temporal poles and middle occipital gyrus, respectively. Finally, from the middle fusiform seed region, a local connection appears to link the fusiform to the inferior temporal gyrus (itg), but only in the left hemisphere. U-fibre Seed regions were manually defined on each participant’s T1 scan using the collateral and occipito-temporal sulci as the medial and lateral borders of the fusiform gyrus, respectively (see next panel) X = 35 inferior temporal gyrus Although the connection between the fusiform gyrus and the inferior temporal gyrus appeared to be left lateralized, this is a preliminary finding in a small sample (n=3) and should be considered as such.