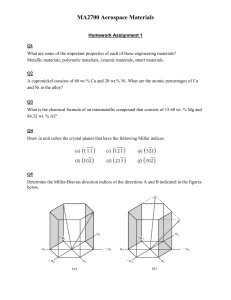
3.1 What is the difference between atomic structure and crystal structure? Unit Cells Metallic Crystal Structures 3.2 If the atomic radius of aluminum is 0.143 nm, calculate the volume of its unit cell in cubic meters. 3.3 Show for the body-centered cubic crystal structure that the unit cell edge length a and the atomic radius R are related through a =4R/ 3 . 3.4 For the HCP crystal structure, show that the ideal c/a ratio is 1.633. 3.5 Show that the atomic packing factor for BCC is 0.68. 3.21 Show that the minimum cation-to-anion radius ratio for a coordination number of 4 is 0.225. 3.23 Demonstrate that the minimum cation-to-anion radius ratio for a coordination number of 8 is 0.732. 3.24 On the basis of ionic charge and ionic radii given in Table 3.4, predict crystal structures for the following materials: (a) CsI, (b) NiO, (c) KI (d) NiS. Justify your selections. 3.25 Which of the cations in Table 3.4 would you predict to form iodides having the cesium chloride crystal structure? Justify your choices. 3.27 The unit cell for Cr2O3 has hexagonal symmetry with lattice parameters a = 0.4961 nm and c = 1.360 nm. If the density of this material is 5.22 g/cm3, calculate its atomic packing factor. For this computation assume ionic radii of 0.062 nm and 0.140 nm, respectively for Cr3+ and O2-. 3.28 Compute the atomic packing factor for cesium chloride using the ionic radii in Table 3.4 and assuming that the ions touch along the cube diagonals. 3.29 Calculate the density of FeO, given that it has the rock salt crystal structure. 3.34 A hypothetical AX type of ceramic material is known to have a density of 2.65 g/cm 3 and a unit cell of cubic symmetry with a cell edge length of 0.43 nm. The atomic weights of the A and X elements are 86.6 and 40.3 g/mol, respectively. On the basis of this information, which of the following crystal structures is (are) possible for this material: rock salt, cesium chloride, or zinc blende? Justify your choice(s). Point Coordinates 3.43 List the point coordinates for all atoms that are associated with the FCC unit cell (Figure 3.1). 3.46 Sketch a tetragonal unit cell , and within that cell indicate locations of the 1 2 1 coordinates. 1 2 and 1 1 3 4 2 4 point 3.51 What are the indices for the directions indicated by the two vectors in the following sketch? 3.52 Within a cubic unit cell, sketch the following directions: (a) [1 10] , (e) [1 1 1] , (b) [1 2 1] , (f) [1 22] , (c) [01 2] , (d) [13 3] , (g) [12 3 ] , (h) [ 1 03] . for the directions shown in the following cubic unit cell: 3.53Determine the indices 3.54 Determine the indices for the directions shown in the following cubic unit cell: Crystallographic Planes 3.60 (a) Draw an orthorhombic unit cell, and within that cell a (210) plane. (b) Draw a monoclinic unit cell, and within that cell a (002) plane. 3.61 What are the indices for the two planes drawn in the following sketch? 3.62 Sketch within a cubic unit cell the following planes: (a) (01 1 ) , (e) (1 11 ) , (b) (112 ) , (f) (12 2 ) , (c) (102 ) , (d) (13 1) , (g) (1 23 ) , (h) (01 3 ) 3.63 Determine the Miller indices for the planes shown in the following unit cell: 3.64 Determine the Miller indices for the planes shown in the following unit cell: 3.72 Convert the (010) and (101) planes into the four-index Miller–Bravais scheme for hexagonal unit cells. 3.74 Sketch the (11 01) and (112 0) planes in a hexagonal unit cell. 3.76 (a) Derive linear density expressions for BCC [110] and [111] directions in terms of the atomic radius R. (b) Compute and compare linear density values for these same two directions for tungsten. 3.78 (a) Derive planar density expressions for BCC (100) and (110) planes in terms of the atomic radius R. (b) Compute and compare planar density values for these same two planes for vanadium. 3.81 The corundum crystal structure found for Al2O3 consists of an HCP arrangement of O2- ions, with the Al3+ ions occupying octahedral positions. (a) What fraction of the available octahedral positions are filled with Al3+ ions? (b) Sketch two close-packed O2–planes stacked in an AB sequence, and note octahedral positions that will be filled with the Al3+ ions. 3.83 Magnesium silicate (Mg2SiO4) forms in the olivine crystal structure that consists of an HCP arrangement of O2ions. (a) Which type of interstitial site will the Mg2+ ions occupy? Why? (b) Which type of interstitial site will the Si4+ ions occupy? Why? (c) What fraction of the total tetrahedral sites will be occupied? (d) What fraction of the total octahedral sites will be occupied?