Document 10619402
advertisement

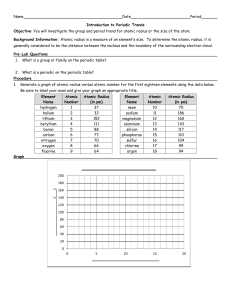
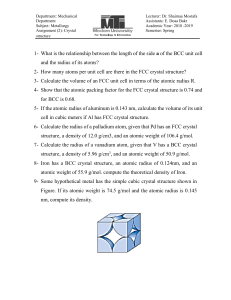
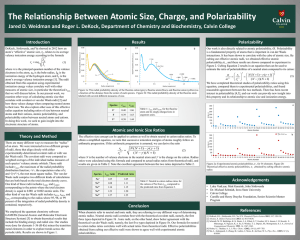
MatE271 HW # 1 Due Friday 8/14/2001 in class No late HW will be accepted, regardless of the reason 1. Explain why the melting temperature of diamond is 10 times higher than Ethylene-molecule based polymers, despite the fact that both have the same type of carbon bond. 2. If the atomic radius of aluminum is 0.143 nm, calculate the volume of its unit cell in cubic meters. 3. Some hypothetical metal has the simple cubic crystal structure shown in Fig. 3.3, pp 61. If its atomic weight is 70.4 g/mol and the atomic radius is 0.126 nm, compute its density. 4. Problem 3.3 pp 111 (Class text; Shackelford, 2001) 5. Problem 3.6 pp 111 (Class text; Shackelford, 2001) 1