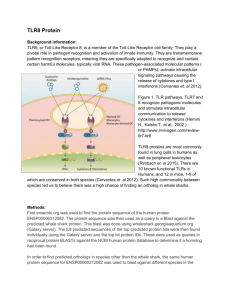
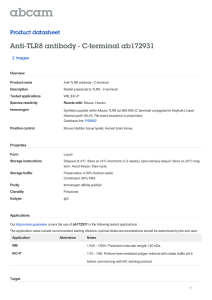
Immunology Letters 261 (2023) 13–16 Contents lists available at ScienceDirect Immunology Letters journal homepage: www.elsevier.com/locate/immlet Human toll-like receptor 8 (TLR8) in NK cells: Implication for cancer immunotherapy Irene Veneziani #, Claudia Alicata #, Lorenzo Moretta *, Enrico Maggi Tumor Immunology Unit, Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy A R T I C L E I N F O A B S T R A C T Keywords: Adjuvants NK cells subsets Endosomal toll like receptors Toll like receptors agonists Tumor immunotherapy Toll-like receptors (TLR)s are homo- or heterodimeric proteins, whose structure and function were widely described in the antigen presenting cells (APC), such as Dendritic cells (DC). Recently, the expression and the role of TLRs in fighting against pathogens, was described also in NK cells. Their activation and functional properties can be directly and indirectly modulated by agonists for TLRs. In particular CD56bright NK cells subset, that is the most abundant NK cell subset in tissues and tumor microenvironment (TME), was mostly activated in terms of pro-inflammatory cytokine production, proliferation and cytotoxicity, by agonists specific for endosomal TLR8. The interplay between DC and NK, that depends on both cell-to-cell contact and soluble factors such as cytokines, promote both DC maturation and NK cell activation. Based on this concept, a TLR based immunotherapy aimed to activate NK-DC axis, may modulate TME by inducing a pro-inflammatory phenotype, thus improving DC ability to present tumor-associated antigens to T cells, and NK cell cytotoxicity against tumor cells. In this minireview, we report data of recent literature about TLRs on human NK cells and their application as adjuvant in cancer vaccines or in combined tumor immunotherapy. 1. Introduction Cells of the innate immunity recognize microbial-associated or pathogen-associated molecular patterns (MAMPs/PAMPs) through their pattern recognition receptors (PRRs) including, NOD-like receptors (NLRs), C-type lectin receptors (CLRs), RIG-I-like receptors (RLRs) and Toll-like receptors (TLRs) [1,2]. Similar to the antigen presenting cells (APC), TLR-mediated signaling pathways represent the first-line of de­ fense against bacterial, viral, and fungal pathogens also in Natural Killer (NK) cells [3–5]. Moreover, TLRs are engaged to favor the cross-talk between NK cells and APC. In particular, plasmacytoid dendritic cells (pDC), which are activated through TLR7/8, promote the release of type I interferon (IFN) [6,7], induce an increased expression of CD69 and potentiate the cytolytic activity of NK cells [8]; on the other hand, activated NK cells stimulate DC maturation, upon the production of TNF-α [8]. This mini-review reports data of recent literature on the expression and function of TLRs on human NK cells and their application in cancer immunotherapy. It will be particularly focused on TLR8 agonists employed as adjuvant in cancer vaccines or in combined tumor immunotherapy, aimed to re-activate intra-tumoral dysfunctional NK cells [9]. 2. Toll-like receptors: features and function TLRs are the most well-defined PRRs expressed on both the cell surface and endosome membranes of immune cells [10,11]. The human TLRs expressed at the cell surface are TLR1, 2, 4, 5, 6, 10 while the endosomal ones are TLR3, 7, 8, 9 [12]. TLRs recognize conserved PAMPs, which serve as TLR agonists/ligands (TLRL), with differences in terms of timing, binding affinity and conformational site of interaction [13,14]. Besides PAMPs, some endogenous components, such as fibrinogen, heat shock proteins, RNA and DNA, also provide signals through TLRs [14,15]. TLRs are expressed not only on cells of innate immunity but also on some cells of the adaptive immunity (as Treg and activated T cells), thus widely contributing to immune response against pathogens [15]. TLRs are type I transmembrane proteins with ectodo­ mains containing leucine-rich repeats (LRRs) that mediate PAMP recognition, transmembrane domains, and a conserved region of ~200 aa intracellular Toll-interleukin 1 (IL-1) receptor (TIR) domain required * Corresponding author at: Bambino Gesù Children’s Hospital, IRCCS, Rome, Italy. E-mail address: Lorenzo.moretta@opbg.net (L. Moretta). # IV and CA equally contributed to the paper. https://doi.org/10.1016/j.imlet.2023.07.003 Received 21 June 2023; Received in revised form 6 July 2023; Accepted 8 July 2023 Available online 13 July 2023 0165-2478/© 2023 The Author(s). Published by Elsevier B.V. on behalf of European Federation of Immunological Societies. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). I. Veneziani et al. Immunology Letters 261 (2023) 13–16 for downstream signal transduction [1,16]. TLRs are homo- or heterodimeric proteins. In particular, TLR8 is present on endosomal membranes, both as homodimer or as hetero­ dimer in combination with TLR7 [17]. TLR8, as well as TLR7, binds single-stranded RNA (ssRNA)-derived from RNA viruses (as vesicular stomatitis, influenza A, and HIV) and imidazoquinoline derivatives as Resiquimod (R848), or guanine analogs as Loxoribine [12,18]. While human TLR7 recognizes GU-rich sequences of ssRNA, human TLR8 binds both AU- and GU-rich sequences thanks to its ability to form ligand-specific secondary structures [9]. Notably, the stimulation of a TLR may modulate the expression of other endosomal TLRs. For instance, TLR8 triggering leads to downregulation of TLR7 and TLR9 and the increase of TLR2 in monocyte/macrophages [19]. This obser­ vation was reproduced in Tlr8− /− mice, in which the absence of TLR8 led to higher levels of expression of TLR7 and interferon-stimulated genes [20]. The intracellular signaling of all TLRs starts with phosphorylation of myeloid differentiation primary response protein88- (MyD88-); the only exception is TLR3 which stimulates the TIR domain-containing adapterinducing interferon-β (TRIF)-dependent pathways [1,16]. Both path­ ways lead to activation of transcription factors as NF-kB, AP1, CREB, IRF3/7, which, in turn, promote the transcription of genes for type I and III interferons (IFN), inflammatory cytokines/chemokines, cos­ timulatory and adhesion molecules and antimicrobial mediators [3]. Clear-cut data indicate that, even though NK cells can be directly activated by some agonists of the endosomal TLRs, both the cross-talk with DCs and the cytokine microenvironment are crucial for activation of NK cell effector function. 4. NK cell subsets are mostly activated through TLR8 When compared to other endosomal TLRL as TLR3L (Poly I:C) and TLR9L (ODN2395), TLR7/8L (R848) resulted the most effective in inducing NK cell activation. Indeed, the proportion of CD69+ and CD25+ NK cells was highly increased upon R848 stimulation, significantly less upon Poly I:C and nothing upon ODN2395 stimulation. In addition, the cytotoxic activity and the cytokine production (IFN-γ, TNF-α, and GMCSF) were improved only with Resiquimod. Importantly, R848 was the only endosomal TLRL able to activate and induce IFN-γ production by CD56brightCD16− and, to a lesser extent, by CD56dimCD16+NK cell subset, while Poly I:C triggered only weakly CD56dimCD16+ subset. Notably, R848 did not induce the increase of CD57 expression on NK cells, suggesting a marginal ability to induce their maturation. Besides induction of activation markers, R848, but not the other TLRL, induced CD56brightCD16− NK cell proliferation (peaking at 4 day) and cytotox­ icity with upregulation of CD107a, Perforin and Granzymes expression [32]. Previous reports underscored the ability of different TLR7/8 agonists (including R848) to stimulate monocytes/macrophages, DC and NK cells; our recent findings indicated that R848 triggered NK cells exclu­ sively through TLR8 [32]. Data from several reports would support this notion, particularly: (i.) TLR8 but not TLR7, is expressed in a dimeric (active) form in a ligand-independent way in NK cells, perhaps favored by DC-driven cytokines [35], (ii.) the IFN− γ produced by NK cells upon different stimuli, is a strong inducer (in an autocrine way) of the expression of TLR8 (much more than TLR7) [36], (iii.) the miRNAs contained in exosomes derived from NK, tumor and tumor environ­ mental cells mainly bind TLR8, impacting on NK cell function [37,38], (iv.) many functions of CD56brightCD16− NK cells are upregulated by the agonists specific for TLR8 (TL8–506) but not for TLR7 (Imiquimod, Loxoribin and modified 8-hydroxy Adenine) [32]. Notably, similar to NK cells, TLR8, but not TLR7, has been recently shown to be functionally active on other innate cells as neutrophils [39,40]. It is relevant to un­ derscore that TLR8 is the only TLR capable of distinguishing the host RNA by nucleoside modifications and of activating its signaling pathway when a microbial RNA enters into the cell [41]. In addition, it is important to underline that TLR8 recognizes microbial structures from viable microbes [42], as well as poly(A)/T sequences and small chemical molecules with antiviral function [43]. 3. Expression of TLRs and their role in NK cell activation The expression of TLRs (especially of endosomal TLRs) in human NK cells is influenced by: (i.) variable degree of activation of freshly-isolated TLR+ NK cells in different donors, (ii.) DC-NK cell interactions mediated by different surface and soluble signals (cytokines) which, in turn, may favor or impair, the expression and triggering of endosomal TLRs in NK cells, (iii.) cross-modulation of such receptors after triggering of one of them, iv. interference of DAMPs and miRNAs with the expression and activation of some TLRs (mainly TLR4 and TLR7/8) [21–25]. The majority of reports agrees that NK cells express functional TLR1 [26], TLR2 [24], TLR3 [22], TLR4 [27], TLR5 [26], TLR7/8 [28,29] and TLR9 [30], regardless their state of activation. mRNA analysis demon­ strated that, in purified NK cells, TLR1 was the most expressed, followed by moderate levels of TLR2, TLR3, TLR5 and TLR6, and low levels of other endosomal TLRs [26,30]. However, there was uncertainty on the expression of different TLRs and activity in the two main NK cell subsets: the CD56dimCD16+ cells which prevail (>90%) in peripheral blood and are specialized in cytotoxic activity, and the CD56brightCD16− cells, which are less cytolytic, produce high levels of cytokines and are less represented in peripheral blood (< 10%), while they are prevalent in tissues (e.g. T-cell areas of lymph nodes) and tumor microenvironment (TME) [31]. A recent study by our group has established that, the endosomal TLRs are consistently expressed in CD56brightCD16− and CD56dimCD16+ NK cell subsets at both the mRNA and protein levels [32]. The response to TLRL occurs in vitro in both activated and freshly isolated NK cells, in which suboptimal doses of inflammatory cytokines, primarily IL-2 and IL-12, generate an increased TLR-mediated activa­ tion. In particular, the agonists of endosomal TLRs can activate NK cell function either directly or indirectly through accessory cells, cytokines or cell-to-cell contact [23]. Recently, we provided definitive evidence that resting NK cells can be activated by endosomal TLRL in the presence of co-stimuli such as suboptimal doses of cytokines, including IL-2, IL-12, IL-15, IL-18 (alone or in combination) [23]. Indeed, some studies indicated that NK cells are activated in vitro by DC-derived IL-12 and T cell-derived IL-2 [22,29,30,33,34]. Taken together, these data reinforce the concept that the direct and indirect impacts of TLR engagement, facilitates the interaction between DC and NK cells which, in turn, may affect downstream other cells of innate and adaptive immunity. 5. Cancer immunotherapy: targeting TLR8 in NK cells Preclinical and clinical studies have provided evidence that the downstream inflammatory response after TLR7 and TLR8 engagement, results in an initial efficacy for the treatment of cancer and viral in­ fections, as well as for its action as vaccine adjuvant. This explains why in the last decade, there has been significant progress in the optimization of novel TLR7 and TLR8 small molecule agonists with more than 70 new patents. These novel compounds are currently analyzed to evaluate their ability to desired immune responses, to improve their safety and to act synergistically with checkpoint inhibitors in combination therapies [44]. When administered intratumorally, TLR8 agonists, in addition to NK cell activation, promote the interplay between NK and macrophages, the M2 to M1 shift, leading to an increased MHC class II expression, and the differentiation of naïve CD4+ T cells into type 1 profile [45]. Moreover, TLR8 agonists mediate the reversal of the suppressive function of human Treg cells through the TLR8-MyD88-IRAK4 signaling pathway and contribute to the follicular helper T cell differentiation [9,42]. Targeting the cross-talk between DC and NK cells may represent a 14 I. Veneziani et al. Immunology Letters 261 (2023) 13–16 Fig. 1. Potential mechanisms of TLR8 agonists in tumor immunotherapy. promising approach to the treatment of infections and cancer. However, it is well-known that the clinical efficacy of TLRL delivered systemically is limited by their solubility, systemic toxicity and possible induction of autoimmunity [46,47]. To overcome these limitations in the use of TLR7/8 agonists in cancer therapy, biomaterial-based drug delivery systems have been developed. These devices can be directly adminis­ tered at the tumor site and are passively accumulated in lymphoid tis­ sues. In addition, they may be triggered by environmental stimuli, and may target specific cell populations. In addition, recent studies reveled the potential benefits of TLR7/8 agonist co-delivery with other thera­ pies, particularly checkpoint inhibitors, experimental cancer vaccines, and chemotherapy, leading to relevant anti-cancer effects [48]. In murine models, the local infusion of endosomal TLR agonists enhanced both the recruitment and the activation of immune cells at the tumor site with more effective NK cell activity, type 1 profile of T cell responses and the efficacy improvement of checkpoint inhibitors [49]. In particular, TLR7/8 agonists have been found to delay tumor growth and even to induce tumor regression in Acute Myeloid Leukemia models [50,51]. Recent studies also showed that some novel TLR7/8 agonists induce robust pro-inflammatory cytokine secretion and enhance NK cell-mediated ADCC in vitro as well as the enhanced efficacy of mono­ clonal antibodies in two different in vivo tumor mouse models [52]. Based on these data, pretreatment of NK cells with endosomal TLRL, or intra-tumoral (IT) therapy with these molecules, may indeed repre­ sent a novel potential strategy of solid tumor therapy [53]. In addition, dermal application of Imiquimod (TLR7 agonist) or Resiquimod (TLR7/8 agonist) to treat human skin tumors, by inducing mainly local activation of NK cells, greatly limits systemic involvement and un­ wanted side-effects [54,55]. Of note, these findings highlight the po­ tential role of TLR8 engagement of NK cells infiltrating TME as a novel strategy of tumor immunotherapy. The predominant activity of TLR8 agonists on CD56brightCD16− NK cells, which are predominant in tissues and in tumor lesions, makes the local infusion of TLR8 agonists more than a potential candidate of cancer therapy. In this context, our recent data showed that TLR8 targeting improved both IFN-γ production and cytotoxic activity of NK cells derived from the ascitic fluid of patients with metastatic ovarian carcinoma (mainly represented by CD56brightCD16− NK cells) [32]. In this tumor, TLR8 signaling activates NK cells which, in turn, may produce cytokines and chemokines which amplify the effect on NK cells, by inducing proliferation and recruitment of other effector cells [32]. In the TME of solid tumors, TLR8 agonists have been reported to induce tumor cell apoptosis and to favor matu­ ration (and loss of function) of myeloid-derived suppressor cells [56] and prevent T cell senescence/exhaustion [57]. Moreover, TLR8 ago­ nists have been reported to exert a metabolic inhibitory effect on CD4+ T regulatory cells present in ovarian cancer cell microenvironment [58]. Lastly, in humans, TLR8 agonists have been locally assayed in clinical phase Ib-II trials in patients with different metastatic solid tumors [49, 59,60]. 6. Concluding remarks In conclusion, recently, different results have suggested that the ef­ fects referred to TLR8 agonists in some in vivo models are consistently mediated by activated NK cells [61,62]. Future research efforts should be devoted to establish which interactions occur between NK cells activated by such agonists and other cells present in the TME, also focusing on drug safety, efficiency and specificity [63]. The findings on TLR8-mediated rescue of NK cell reactivity in ascites of patients with ovarian cancer, can also be exploited as a new in vitro assay to determine which patients could obtain relevant benefits by the therapy with infused TLR8 agonists in the peritoneal cavity (as adjuvants of tumor vaccines or combined chemo- or immuno-therapies) and/ or the adop­ tive transfer of autologous or allogenic NK cells expanded in vitro with TLR8 ligands (Fig. 1). 15 I. Veneziani et al. Immunology Letters 261 (2023) 13–16 Declaration of Competing Interest [30] S. Sivori, et al., CpG and double-stranded RNA trigger human NK cells by Toll-like receptors: induction of cytokine release and cytotoxicity against tumors and dendritic cells, Proc. Natl Acad. Sci. U S A, 101 (27) (2004) 10116–10121. [31] T.A. Fehniger, et al., CD56bright natural killer cells are present in human lymph nodes and are activated by T cell-derived IL-2: a potential new link between adaptive and innate immunity, Blood 101 (8) (2003) 3052–3057. [32] I. Veneziani, et al., Toll-like receptor 8 agonists improve NK-cell function primarily targeting CD56(bright)CD16(-) subset, J. Immunother. Cancer 10 (1) (2022). [33] S. Sivori, et al., Heterogeneity of TLR3 mRNA transcripts and responsiveness to poly (I:C) in human NK cells derived from different donors, Int. Immunol. 19 (12) (2007) 1341–1348. [34] S. Sivori, et al., Comparison of different CpG oligodeoxynucleotide classes for their capability to stimulate human NK cells, Eur. J. Immunol. 36 (4) (2006) 961–967. [35] K. Maeda, S. Akira, TLR7 Structure: Cut in Z-Loop, Immunity 45 (4) (2016) 705–707. [36] M.P. Gantier, et al., Genetic modulation of TLR8 response following bacterial phagocytosis, Hum. Mutat. 31 (9) (2010) 1069–1079. [37] M. Fabbri, TLRs as miRNA receptors, Cancer Res. 72 (24) (2012) 6333–6337. [38] M. Fabbri, et al., MicroRNAs bind to Toll-like receptors to induce prometastatic inflammatory response, Proc. Natl Acad. Sci. U S A, 109 (31) (2012) E2110–E2116. [39] M.A. Cassatella, et al., Human neutrophils activated by TLR8 agonists, with or without IFNgamma, synthesize and release EBI3, but not IL-12, IL-27, IL-35, or IL39, J. Leukoc. Biol. 108 (5) (2020) 1515–1526. [40] N. Tamassia, et al., Human neutrophils activated via TLR8 promote Th17 polarization through IL-23, J. Leukoc. Biol. 105 (6) (2019) 1155–1165. [41] K. Kariko, et al., Suppression of RNA recognition by Toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA, Immunity 23 (2) (2005) 165–175. [42] M. Ugolini, et al., Recognition of microbial viability via TLR8 drives T(FH) cell differentiation and vaccine responses, Nat. Immunol. 19 (4) (2018) 386–396. [43] U. Ohto, H. Tanji, T. Shimizu, Structure and function of toll-like receptor 8, Microbes Infect. 16 (4) (2014) 273–282. [44] M.E. Kieffer, et al., Small molecule agonists of toll-like receptors 7 and 8: a patent review 2014 - 2020, Expert Opin. Ther. Pat. 30 (11) (2020) 825–845. [45] M. de Marcken, et al., TLR7 and TLR8 activate distinct pathways in monocytes during RNA virus infection, Sci. Signal. 12 (605) (2019). [46] A.Z. Dudek, et al., First in human phase I trial of 852A, a novel systemic toll-like receptor 7 agonist, to activate innate immune responses in patients with advanced cancer, Clin. Cancer Res. 13 (23) (2007) 7119–7125. [47] B.K. Link, et al., Oligodeoxynucleotide CpG 7909 delivered as intravenous infusion demonstrates immunologic modulation in patients with previously treated nonHodgkin lymphoma, J. Immunother. 29 (5) (2006) 558–568. [48] D. Varshney, et al., Employing drug delivery strategies to overcome challenges using TLR7/8 agonists for cancer immunotherapy, AAPS J. 23 (4) (2021) 90. [49] C.P. Belani, et al., A randomized trial of TLR-2 agonist CADI-05 targeting desmocollin-3 for advanced non-small-cell lung cancer, Ann. Oncol. 28 (2) (2017) 298–304. [50] J.J. Ignatz-Hoover, et al., The role of TLR8 signaling in acute myeloid leukemia differentiation, Leukemia 29 (4) (2015) 918–926. [51] S.R. Mullins, et al., Intratumoral immunotherapy with TLR7/8 agonist MEDI9197 modulates the tumor microenvironment leading to enhanced activity when combined with other immunotherapies, J. Immunother. Cancer 7 (1) (2019) 244. [52] V. Khanna, et al., Novel TLR 7/8 agonists for improving NK cell mediated antibodydependent cellular cytotoxicity (ADCC), Sci. Rep. 11 (1) (2021) 3346. [53] S.K. Gadkaree, et al., Induction of tumor regression by intratumoral STING agonists combined with anti-programmed death-L1 blocking antibody in a preclinical squamous cell carcinoma model, Head Neck 39 (6) (2017) 1086–1094. [54] A.H. Rook, et al., Topical resiquimod can induce disease regression and enhance Tcell effector functions in cutaneous T-cell lymphoma, Blood 126 (12) (2015) 1452–1461. [55] M. Schon, et al., Tumor-selective induction of apoptosis and the small-molecule immune response modifier imiquimod, J. Natl Cancer Inst. 95 (15) (2003) 1138–1149. [56] H. Kim, et al., Combination of sunitinib and PD-L1 blockade enhances anticancer efficacy of TLR7/8 agonist-based nanovaccine, Mol. Pharm. 16 (3) (2019) 1200–1210. [57] J. Ye, G. Peng, Controlling T cell senescence in the tumor microenvironment for tumor immunotherapy, Oncoimmunology 4 (3) (2015), e994398. [58] B.J. Monk, et al., A phase 2, randomized, double-blind, placebo- controlled study of chemo-immunotherapy combination using motolimod with pegylated liposomal doxorubicin in recurrent or persistent ovarian cancer: a Gynecologic Oncology Group partners study, Ann. Oncol. 28 (5) (2017) 996–1004. [59] L.Q.M. Chow, et al., Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN, Clin. Cancer Res. 23 (10) (2017) 2442–2450. [60] M.R. Weihrauch, et al., Phase I clinical study of the toll-like receptor 9 agonist MGN1703 in patients with metastatic solid tumours, Eur. J. Cancer 51 (2) (2015) 146–156. [61] C.L. Chiang, L.E. Kandalaft, In vivo cancer vaccination: which dendritic cells to target and how? Cancer Treat. Rev. 71 (2018) 88–101. [62] E.M. Doorduijn, et al., CD4(+) T cell and NK cell interplay key to regression of MHC class I(low) tumors upon TLR7/8 agonist therapy, Cancer Immunol. Res. 5 (8) (2017) 642–653. [63] X. Huang, X. Zhang, M. Lu, Recent trends in the development of Toll-like receptor 7/8-targeting therapeutics, Expert Opin. Drug Discov. 16 (8) (2021) 869–880. The authors declare that they have no conflicts of interest. Acknowledgements This work was supported by the Italian Ministry of Health with “Current Research funds” (grant no. RC-2020 OPBG to L.M. and E.M.) and from Associazione Italiana per la Ricerca sul Cancro (project no. 5×1000 2018 Id 21147 and project no. IG 2017 Id 19920 to L.M.). I.V. was supported by FIRC-AIRC fellowship for Italy; C.A.is a recipient of a grant awarded by Fondazione Umberto Veronesi. References [1] T. Kawai, S. Akira, The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors, Nat. Immunol. 11 (5) (2010) 373–384. [2] M. Yoneyama, T. Fujita, RNA recognition and signal transduction by RIG-I-like receptors, Immunol. Rev. 227 (1) (2009) 54–65. [3] M. Adib-Conquy, et al., TLR-mediated activation of NK cells and their role in bacterial/viral immune responses in mammals, Immunol. Cell Biol. 92 (3) (2014) 256–262. [4] M. Della Chiesa, et al., Human NK cell response to pathogens, Semin. Immunol. 26 (2) (2014) 152–160. [5] E. Vivier, et al., Innate or adaptive immunity? The example of natural killer cells, Science 331 (6013) (2011) 44–49. [6] V. Salvi, et al., SARS-CoV-2-associated ssRNAs activate inflammation and immunity via TLR7/8, JCI Insight 6 (18) (2021). [7] A. Moretta, Natural killer cells and dendritic cells: rendezvous in abused tissues, Nat. Rev. Immunol. 2 (12) (2002) 957–964. [8] F. Gerosa, et al., The reciprocal interaction of NK cells with plasmacytoid or myeloid dendritic cells profoundly affects innate resistance functions, J. Immunol. 174 (2) (2005) 727–734. [9] I. Martinez-Espinoza, A. Guerrero-Plata, The Relevance of TLR8 in Viral Infections, Pathogens 11 (2) (2022). [10] C.C. Lee, A.M. Avalos, H.L. Ploegh, Accessory molecules for Toll-like receptors and their function, Nat. Rev. Immunol. 12 (3) (2012) 168–179. [11] L.A. O’Neill, D. Golenbock, A.G. Bowie, The history of Toll-like receptors redefining innate immunity, Nat. Rev. Immunol. 13 (6) (2013) 453–460. [12] S. Akira, S. Uematsu, O. Takeuchi, Pathogen recognition and innate immunity, Cell 124 (4) (2006) 783–801. [13] S. Akira, K. Takeda, Toll-like receptor signalling, Nat. Rev. Immunol. 4 (7) (2004) 499–511. [14] B. Beutler, Inferences, questions and possibilities in Toll-like receptor signalling, Nature 430 (6996) (2004) 257–263. [15] I. Vaknin, et al., A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression, Blood 111 (3) (2008) 1437–1447. [16] Y. Xu, et al., Structural basis for signal transduction by the Toll/interleukin-1 receptor domains, Nature 408 (6808) (2000) 111–115. [17] S. Zhang, et al., Small-molecule inhibition of TLR8 through stabilization of its resting state, Nat. Chem. Biol. 14 (1) (2018) 58–64. [18] T. Kawai, S. Akira, Innate immune recognition of viral infection, Nat. Immunol. 7 (2) (2006) 131–137. [19] M. Muzio, et al., Differential expression and regulation of toll-like receptors (TLR) in human leukocytes: selective expression of TLR3 in dendritic cells, J. Immunol. 164 (11) (2000) 5998–6004. [20] A.M. Paul, et al., TLR8 couples SOCS-1 and restrains TLR7-mediated antiviral immunity, exacerbating West Nile virus infection in mice, J. Immunol. 197 (11) (2016) 4425–4435. [21] S. Agaugue, et al., Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differently modulate priming of naive T cells by monocyte-derived dendritic cells, Blood 112 (5) (2008) 1776–1783. [22] G. Alter, et al., Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells, J. Immunol. 178 (12) (2007) 7658–7666. [23] M. Della Chiesa, et al., Pathogen-induced private conversations between natural killer and dendritic cells, Trends Microbiol. 13 (3) (2005) 128–136. [24] E. Marcenaro, et al., Human NK cells directly recognize Mycobacterium bovis via TLR2 and acquire the ability to kill monocyte-derived DC, Int. Immunol. 20 (9) (2008) 1155–1167. [25] B. Morandi, et al., NK cells of human secondary lymphoid tissues enhance T cell polarization via IFN-gamma secretion, Eur. J. Immunol. 36 (9) (2006) 2394–2400. [26] J.Y. Noh, et al., Toll-like receptors in natural killer cells and their application for immunotherapy, J. Immunol. Res. 2020 (2020), 2045860. [27] C. Mestre-Duran, et al., Ruxolitinib does not completely abrogate the functional capabilities of TLR4/9 ligand-activated NK cells, Front. Immunol. 13 (2022), 1045316. [28] A. Chalifour, et al., Direct bacterial protein PAMP recognition by human NK cells involves TLRs and triggers alpha-defensin production, Blood 104 (6) (2004) 1778–1783. [29] O.M. Hart, et al., TLR7/8-mediated activation of human NK cells results in accessory cell-dependent IFN-gamma production, J Immunol 175 (3) (2005) 1636–1642. 16