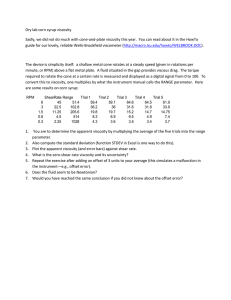
RESEARCH ARTICLE | SEPTEMBER 25 2017
The determination of viscosity at liquid mixtures –
Comparison of approaches
Schmirler Michal; Netřebská Hana; Kolínský Jan
AIP Conf. Proc. 1889, 020035 (2017)
https://doi.org/10.1063/1.5004369
View
Online
CrossMark
Export
Citation
14 January 2024 10:30:30
7KH'HWHUPLQDWLRQRI9LVFRVLW\DW/LTXLG0L[WXUHV±
&RPSDULVRQRI$SSURDFKHV
Schmirler Michal1, a) and Netřebská Hana2,b) and Kolínský Jan3, c)
1,2
CTU in Prague, Faculty of Mechanical Engineering, Department of Fluid Dynamics and Thermodynamics,
Technicka 4, 16607 Prague, Czech Republic
3
The Institute of Technology and Business in České Budějovice, Okruzni 517/10, 37001 Ceske Budejovice
a)
Corresponding author: michal.schmirler@fs.cvut.cz
b)
hana.netrebska@fs.cvut.cz
c)
kolinsky@mail.vstecb.cz
,1752'8&7,21
Many industrial applications require knowledge of liquid viscosities, for example, for optimizing chemical
processes and determining power supplies associated with pipeline transportation and pump operations. Glycerol
solutions are widely used in experimental studies of flow phenomena. The theory for determining the viscosity of
liquids and its solutions is still a poor discovered area. Some theoretical approaches including those developed based
on molecular dynamics could provide valuable understandings of relevant fundamentals but often cause large
deviations from measured viscosity data [1,2]. Practically, calculation of the mixture viscosity is often performed with
empirical data-driven correlations between viscosity and other liquid properties. Such correlations are generally
interpolative. Some are simple but applicable to limited conditions, while the others may involve application
procedures that are tedious
Various theoretical approaches for determining the viscosity of the liquid mixtures have been compared in this
paper. These theoretical approaches were compared with data measured by a rotating viscometer. A mixture of
glycerol and water was used to compare the individual results.
36th Meeting of Departments of Fluid Mechanics and Thermodynamics
AIP Conf. Proc. 1889, 020035-1–020035-5; https://doi.org/10.1063/1.5004369
Published by AIP Publishing. 978-0-7354-1572-0/$30.00
020035-1
14 January 2024 10:30:30
$EVWUDFW The research of flow field parameters for non-stationary flow of non-Newtonian fluids carried out at the
Institute of Fluid Mechanics and Thermodynamics of CTU showed the need for knowledge of determination of the
resulting viscosity of a mixture of several liquids. There are several sources for determining viscosity of mixtures. It
is possible either to find theoretical relations in the literature or use technical tables based on experimentally measured
data. This article focuses on comparing these approaches with an experiment. The experiment was performed by a
Rheotest RN 4.1 rotating viscometer produced by the company RHEOTEST Medingen. The research was carried out
using a solution of glycerol and water. The research has shown great differences in results in different approaches for
determining the viscosity of the liquid mixtures. The result of this paper is to determine the method of viscosity
calculation that is closest to the experimental data.
(;3(5,0(17$/'$7$
The RN 4.1 rotating viscometer built by company Rheotest Medingen was used for all the measurements (Fig.1).
This rotary viscometer can be used both in the rotary cylinder configuration and in the rotating plate configuration.
Thanks to the rich accessory, this viscometer can be used to measure the viscosity of liquids in the range of 3mPa.s to
105mPa.s. Unfortunately, clean water and other low viscosity fluids can not be measured with this viscometer.
Capillary or falling body viscometers must be used to measure these fluids. Within this article, only concentrations
that could be evaluated using this rotary viscometer were measured. Practically it means a mixtures of glycerol with
a weight fraction greater than 50%.
),*85(. Rotational viscometer RN 4.1 Rheorest
),*85(Measured results; values of dynamic viscosity of different mass
concentrations of glycerol. Uncertainty of all measured data was lower than 10 -4 Pa.s.
020035-2
14 January 2024 10:30:30
The dynamic viscosity of the glycerol / water mixture was measured at 20 ° C and 30 °C. Various weight
concentrations of glycerol in water were used. Gradually, samples were mixed with a weight percentage of 60%
glycerol to 100% glycerol in water. The measured results are shown in the graph in Figure 2.
7+(25(7,&$/'$7$
The measured results were compared with several theoretical approaches found in the literature. For example,
Chen and Pearlstein [3] proposed a four-parameter correlation for the dynamic viscosity (μ) of glycerol-water
mixtures:
ߤ ൌ ܣଵ ݁ݔሾܣଶ ሺܶ ʹ͵Ǥͳͷሻିଷ ܣଷ ሺܶ ʹ͵Ǥͳͷሻ ܣସ ሺܶ ʹ͵Ǥͳͷሻିଵ ሿ ,
(1)
where μ is in mPa.s or 0.001Ns/m2, T is in °C, and the coefficients, A1 to A4, vary with the mass concentration of
glycerol [4]. Use of Eq. (1) is restricted because the values of A1 to A4 are only given for some discrete concentrations,
i.e. Cm = 40%, 50%, 90%, 99%, 100%. For other concentrations, numerical interpolations or extrapolations are
necessary, which may induce additional computational errors. Shankar and Kumar [5] suggested the kinematic
viscosity (ߥ) of aqueous glycerol solutions can be estimated as:
୪୬ሺఔȀఔೢ ሻ
୪୬ሺఔ Ȁఔೢ ሻ
ଶ ሻሿ,
ൌ ܥ ሾͳ ሺͳ െ ܥ ሻሺܤଵ ܤଶ ܥ ܤଷ ܥ
(2)
ߤൌ
௫భ ఘభ ା௫మ ఘమ
ഐ
ഐ ௫భ భ ା௫మ మ
ഋభ
,
(3)
ഋమ
where ݔଵ and ݔଶ are volume concentrations of the two pure substances in the mixture and ߩଵǡଶ ǡ ߤଵǡଶ . are their densities
and viscosities. Authors report accuracy to 1% for mixtures (ܰܽ ݈ܥെ ܰܪସ ݈ܥെ ܪଶ ܱǡ ܰܽ ݈ܥെ ݈ܥܭെ ܪଶ ܱǡ ܱܰܽ ܪെ
ܰܽଶ ܱܥଷ െ ܪଶ ܱǡ ܰܽ ݈ܥെ ݈ܥ݃ܯଶ െ ܪଶ ܱ) and 6-9% for solutions of݈ܥܭǡ ݈ܥܪǡ ݈ܥ݅ܮǡ ݈ܰܽܥǡ ܰܪସ ݈ܥ.
In addition, the book [8] describes the methods of calculating using the third roots:
ߤ ଵȀଷ ൌ ݔଵ ߤଵ ݔଶ ߤଶ
,
(4)
where ݔଵ and ݔଶ are the mass or better molar concentrations of pure components of the mixture. Another possibility
of calculation is the use of natural logarithms:
ଵȀଷ
ଵȀଷ
ሺߤሻ ൌ ݔଵ ሺߤଵ ሻ ݔଶ ሺߤଶ ሻ ݔଵ ݔଶ ݀,
(5)
where the last member could be used. The coefficient d in Eq. (4) includes the mutual forces of the individual
components of the mixture. The best agreement with the experiment was achieved using ݔଵ and ݔଶ as the mass
fractions and the ݔଵ and ݔଶ as the molar concentrations together with the coefficient ݀ ൌ െ͵.
There are many other ways to describe the viscosity behaviour of a mixture of liquids. More accurate methods are
based on the evaluation of the bonding forces between the molecules. Examining these dependencies was not the
subject of this article.
020035-3
14 January 2024 10:30:30
where subscripts g and w denote glycerol and water, respectively, and B1 to B3 are the temperature-dependent
coefficients. Similar difficulties are encountered in applying Eq. (2) because the B-coefficients were evaluated only
for five discrete temperatures, i.e. T = 10, 20, 30, 40, 50°C. These calculations agree quite accurately with experimental
data. Unfortunately, these and other calculations in this article are valid only for glycerol / water mixture.
Online calculations based on [3] can be found in the Internet, for example [6]. Results of this calculator were also
included into comparing.
The technical tables written by Prof. Jiří Šesták in 1981 [6] were used as an additional source of determination of
the resulting viscosity of the mixture of glycerol and water. The values of the dynamic viscosity of glycerol / water
mixture can be found for various weight concentrations of glycerol and water and for 20 °C, 25 °C and 30 °C in these
tables. The properties of many other substances relevant to the engineering of the transport processes can be found in
this literature.
As a last source for the calculation of the resulting viscosity of the mixture of liquids, the publication Gasses and
liquids properties [8] was used. Analytical viscosity calculations for different liquid mixtures are described here. For
example, for salt solutions in water the following relationship can be used:
&203$5,6212)7+(25(7,&$/$1'(;3(5,0(17$/'$7$
The data obtained by computing the above sources were compared with experimental viscosity measurements as
the next step. A mixture of glycerol and water at 20°C was used for all comparisons. The comparison results are shown
in the figure 3. It can be shown that the method using the power law is the most distant of experimental data. In
contrast, the best agreement with experimental data showing the value calculated according to Chen and Pearlstein
[3]. These values also coincide with the values listed in the technical tables (Sestak & coll. [7]). Very good agreement
report values calculated using the natural logarithm. The results show better agreement using molar concentrations
instead of mass concentrations.
The best results were obtained using the logarithmic dependence supplemented by the correction coefficient d. In
this case, better results were obtained by using mass concentrations instead of the molar concentrations. The
dependence of the dynamic viscosity of the mixture on the weight concentration of glycerol for the different values of
the coefficient d shows the following graph (Figure 4).
),*85( Comparison of calculation results using logarithmic dependence for individual
coefficients d. Best matches are achieved using d = -3.
020035-4
14 January 2024 10:30:30
),*85(Comparing the individual results of the viscosity calculation with the experimental data. &21&/86,216
Various methods of calculating the viscosity of liquid mixtures were compared with the experimental data in this
article. The results of the theoretical calculations were compared with the results of the viscosity measurements of the
glycerol / water mixture using the rotational viscometer RN 4.1 Rheotest. The calculations showed a great variance
of values. Many relationships reported in the literature are based on empirically determined dependencies and are
therefore only applicable to a particular fluid mixture. More general relationships involve binding energy between
molecules of substances and their use is already more complex. The sources found for the glycerol-water mixture are
accurate and give good mutual agreement [3, 6, 7]. More general relationships, easy to use, have been found in [8].
This is in particular the use of logarithmic dependence supplemented by the correction coefficient. The data thus
obtained corresponded very well with the experiment. A correction factor was determined for the mixture. This factor
has to be found for a given fluid mixture, which will be the cause of future research.
$&.12:/('*(0(17
Authors would like to thank to Mr. prof. Ing. Pavel Šafařík, CSc. for very useful advices and to the Institute of
Technology and Business in České Budějovice for the providing of the rotational viscometer.
5()(5(1&(6
020035-5
14 January 2024 10:30:30
1. D.S.Viswanath and T.K.Ghosh, Viscosity of liquids: theory, estimation, experiment, and data, (Springer:
Dordrecht, 2007.)
2. B.E.Poling and J.M. Prausnitz, The properties of gases and liquids, 5th ed.; (McGraw-Hill: New York, 2001.)
3. N.S.Chen, “Formula for the Viscosity of a Glycerol−Water Mixture”, Industrial & Engineering Chemistry
Research, , (9), pp. 3285–3288, 2008.
4. Y.M.Chen and A.J. Pearlstein, “Viscosity temperature correlation for glycerol water solutions”, Industrial &
Engineering Chemistry Research, , (8), pp.1670-1672, 1987.
5. P.N.Shankar and M. Kumar, “Experimental-determination of the kinematic viscosity of glycerol water
mixtures”, Proceedings of the Royal Society of London Series a-Mathematical Physical and Engineering Sciences
444, (1922), 573-581, 1994.
6. On-line web: http://www.met.reading.ac.uk/~sws04cdw/viscosity_calc.html
7. J. Šesták and coll., Transportní a termodynamická data pro výpočet aparátů a strojního zařízení, ČVUT,
Praha, 1981.
8. П. Г. Романкова, Свойства газов и жидкостей, Москва, 1966.