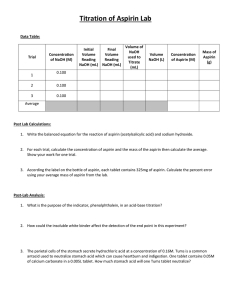
Titration of Aspirin A titration is a lab process where substances are combined using volumetric glassware, such as buret, in a carefully controlled way such that the exact amounts needed to react are used. You already know from the Bonding Lab, that aspirin is weak electrolyte. Aspirin is a weak electrolyte because it is weakly acidic. Commercially available aspirin tablets have aspirin and an inert binder to hold the tablet together. Your task will be to determine how much aspirin is in the tablet and how much of the inert binder is in the tablet. Aspirin (acetyl salicylic acid C9H8O4, abbreviated HAS) reacts with sodium hydroxide according to the following equation: HAS(aq) + NaOH(aq) H2O(ℓ) + NaAS(aq) In the Lab: 1. Burets are probably the single most expensive piece of equipment in the lab. Be exceedingly careful with the burets. 2. One of the difficulties associated with NaOH is that it tends to react with atmospheric carbon dioxide: NaOH + CO2 NaHCO3. This presents a problem because the amount of NaHCO3 will cause a concentration calculated strictly from the mass of NaOH used to make the solution to be higher than the actual concentration. To account for how much sodium hydroxide is actually dissolved in solution, Mrs. Atkins will titrate the NaOH solution with a primary standard, potassium hydrogen phthalate, KHC8H4O4 (abbreviated KHP). You will use the information provided from this standardization to calculate the concentration of NaOH solution you used. NaOH reacts with the KHP according to the following equation: NaOH + KHC8H4O4 H2O + NaKC8H4O4 3. Your buret will have been properly prepared by Mrs. Atkins. Fill the buret with NaOH solution. It is a waste of time to try to get the liquid level exactly on the 0.00mL line. You only need to record the exact position of the liquid line and you can determine the volume added by difference. Burets can be accurately read to 2 decimal places at the bottom of the meniscus. 4. Find the mass of an aspirin tablet using the electronic balances. Place this aspirin tablet in a 250mL Erlenmeyer flask. Add roughly 20mL of distilled water to the Erlenmeyer flask. Wait around thirty seconds for the aspirin tablet to swell and fall apart. After the tablet falls apart, add roughly 20mL of ethanol to dissolve the aspirin. Swirl the solution to ensure that it is homogenous. There is likely to be undissolved binder remaining in the flask. This is ok. 5. Add 5 drops of phenolphthalein indicator to the flask. Phenolphthalein turns pink in basic solutions. 6. Slowly titrate the aspirin with the NaOH. You should vigorously swirl (not shake) the flask continuously to ensure the solution is homogeneous. The end point is the faintest possible pink color that you can perceive. Ideally, one drop of NaOH should change your solution from colorless to bright pink. This is rather rare. You should focus on getting the lowest possible level of pink you can. Record your final volume of NaOH added. 7. Pour your mixture in the Erlenmeyer flask down the drain with lots of water. 8. Repeat the procedure with a second aspirin tablet. You must get at least two good trials. This means that you will probably do more than just two trials. 9. Mrs. Atkins will properly clean your buret. Lab Report: A) Purpose B) Analysis Questions 1) Show your calculations for the molarity of the NaOH from each of the three standardizations. 2) Show your calculation for the average molarity of the NaOH based on the standardization. 3) Show your calculations for the mass of aspirin that reacted in each of the two trials based on the amount of NaOH used. 4) Show your calculations for the average mass of aspirin in a tablet of aspirin. 5) Show your calculations for the percentage of an aspirin tablet that is aspirin. 6) Aspirin tablets are manufactured to contain 325mg of aspirin. Calculate your percent error in your mass of aspirin in a tablet: % Error Experimental Theoretical Theoretical 100 7) Suggest two specific errors that would account for your specific error in your values of aspirin in an aspirin tablet and explain how these errors would affect your values. You may NOT say generic things like human error or inaccuracies of the balance. You must instead come up with specific things that were done in lab that could account for your values not being exactly 325mg. You must then explain how these factors affect your results. If you got exactly 325mg on all your trials then give one reason why someone might have gotten a value above 325mg and one reason for a value below 325mg. C) Conclusions D) Original Data Sheet including standardization data