ProbSet17
advertisement
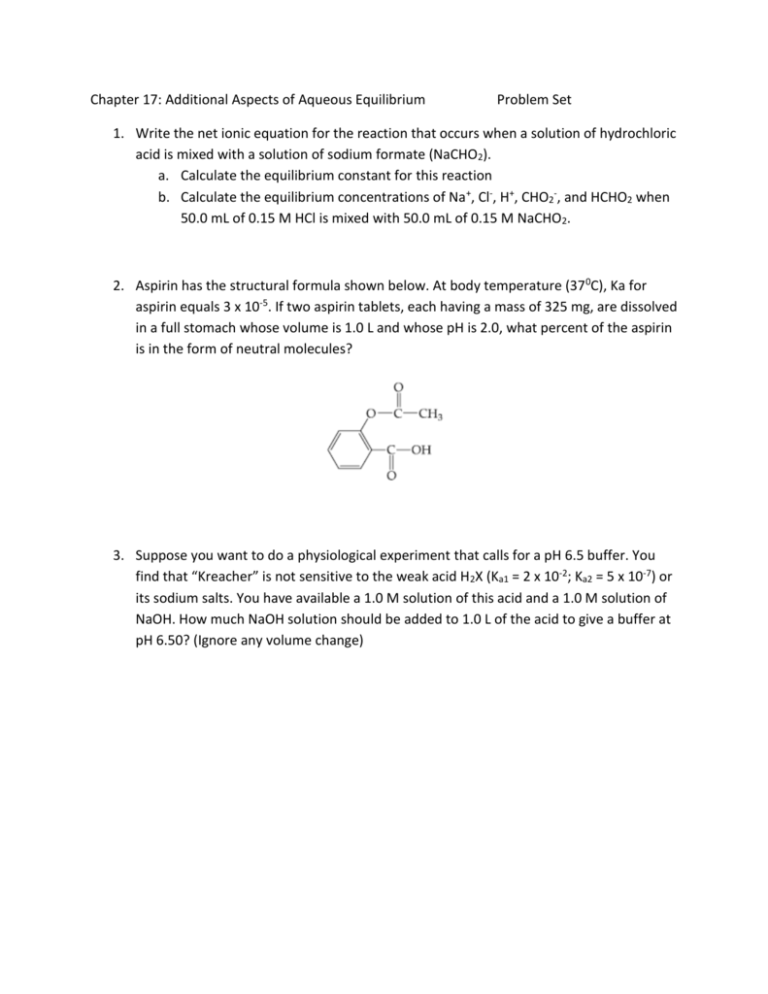
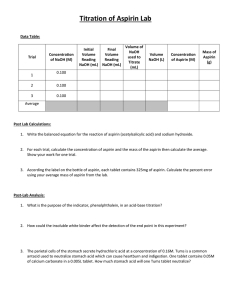
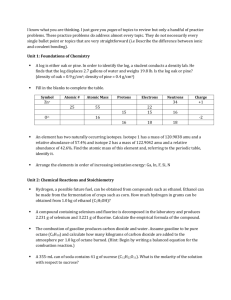
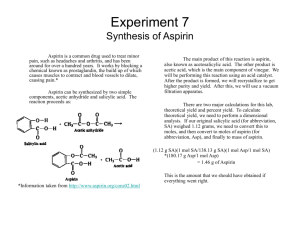
Chapter 17: Additional Aspects of Aqueous Equilibrium Problem Set 1. Write the net ionic equation for the reaction that occurs when a solution of hydrochloric acid is mixed with a solution of sodium formate (NaCHO2). a. Calculate the equilibrium constant for this reaction b. Calculate the equilibrium concentrations of Na+, Cl-, H+, CHO2-, and HCHO2 when 50.0 mL of 0.15 M HCl is mixed with 50.0 mL of 0.15 M NaCHO2. 2. Aspirin has the structural formula shown below. At body temperature (37 0C), Ka for aspirin equals 3 x 10-5. If two aspirin tablets, each having a mass of 325 mg, are dissolved in a full stomach whose volume is 1.0 L and whose pH is 2.0, what percent of the aspirin is in the form of neutral molecules? 3. Suppose you want to do a physiological experiment that calls for a pH 6.5 buffer. You find that “Kreacher” is not sensitive to the weak acid H2X (Ka1 = 2 x 10-2; Ka2 = 5 x 10-7) or its sodium salts. You have available a 1.0 M solution of this acid and a 1.0 M solution of NaOH. How much NaOH solution should be added to 1.0 L of the acid to give a buffer at pH 6.50? (Ignore any volume change)