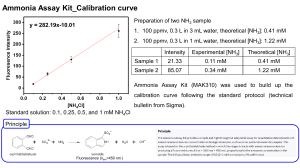
V.SANTHANAM Department of Chemistry SCSVMV Complexes in which exchange of one or more ligands are rapidly exchanged are called labile complexes. If the rate of ligand exchange is slow then the complex is said to be inert. Lability is not related to the thermodynamic stability of a complex. A stable complex may be labile or inert , so as the unstable complex . [Cu(NH3)4(H2O2)2]2+ is labile. solution is blue in color. Its aqueous When concentrated hydrochloric acid is added to this solution, the blue solution immediately turns green ,giving [CuCl4] 2-. But when the complex is kept as such it remains as such with out any decomposition (i.e stable) [Co(NH3)6]3+ reacts slowly. When this complex is treated with concentrated HCl, no reaction takes place. Only when it is heated with 6M HCl for many hours, one NH3 is substituted by Cl-. [Co(NH3)6]3++ HCl [Co(NH3)5Cl]2+ + NH4+ Size of the central metal ion Smaller the size of the metal ion, greater will be the inertness because the ligands are held tightly by the metal ion. Charge on the central metal ion Greater the charge on the metal ion, greater will be the inertness of the complex. Since the M-L bonds are stronger. d-electron configuration If electrons are present in the antibonding eg* orbitals, the complex will be labile -the ligands will be weakly bonded to the metal and hence can be substituted easily. Complexes with empty t2g orbitals, will be labile because ligands can approach easily without much repulsion. In short, if the complex contains less than three delectrons, it will be labile. Or, if one or more eg* electrons are present, it will be labile No. of d electrons & electron configuration Nature Example d0 d1; t2g1eg0 Labile [CaEDTA]2- Labile [Ti(H2O)6]3+ d2; t2g2eg0 Labile [V(phen)3]3+ d3; t2g3eg0 d4(high-spin); t2g3eg1 Inert [V(H2O)6] 3+ Labile d4(low-spin); t2g4eg0 d5(high-spin); t2g3eg2 [Cr(H2O)6]3+ Inert [Cr(CN)6]4- Labile [Mn(H2O)6]2+ d5(low-spin); t2g5eg0 Inert [Mn(CN)6]4- d6(high-spin); t2g4eg2 Inert [Mn(H2O)6]2+ d6(low-spin); t2g6eg0 Inert [Fe(CN)6]4- d7, d8, d9, d10 Labile CFT assumes the splitting of d orbitals of metal. Filling of e- s in them results in different CFSE. CFAE – Crystal Field Activation Energy CFAE = CFSE of intermediate – CFSE of Reactant Since the geometries of the reactant and intermediate are different their splitting and CFSE are also different. If the calculated CFAE is negative or zero or low the reacting complex will require less energy to form the intermediate, hence it will be labile. If CFAE is a high positive value then the complex will be inert. It must be borne in mind that CFAE is only a part of actual AE and other factors are also operative. The geometry of the complex is assumed to be Oh even if all the ligands are not identical. The inter electronic repulsions are neglected. The Dq values of the reactant intermediate are assumed to be same. The Jahn-Teller effect is not affecting CFSE. and Because of the drastic assumptions made, some of the CFAE values are –ive. However when calculated with proper attention to all effects, CFAE is always +ive. CFAE can be small or zero but never –ive By oversimplified approach the –ive values of CFAE may be taken as zero. Substitution of ligands Solvolysis Anation Reactions of coordinated ligands Racemization Electron transfer reactions Photo chemical reactions • Ligand displacements are nucleophilic substitution reactions. • Rate is governed by ligand nucleophilicity The rate of attack on a complex by a given ligand relative to the rate of attack by a reference base. 8 Three types of ligands are present – Entering Ligand: Y – Leaving Ligand: X – Spectator Ligand • Species that neither enters nor leaves • Particularly important when located in a Trans position, designated T Dissociative: One of the ligands dissociates from the reactant, to form a reaction intermediate with lower coordination number than reactants or products • Octahedral complexes and smaller metal centers • Rates depend on leaving group SYSTE M Weak Field / High Spin Strong Field / Low Spin Oh SP CFAE Oh SP CFAE d0 0 0 0 0 0 0 d1 -4 -4.57 -0.57 -4 -4.57 -0.57 d2 -8 -9.14 -1.14 -8 -9.17-4 -1.14 d3 -12 -10.00 2.00 -12 -10.00 2.00 d4 -6 -9.14 -3.14 -16 -14.57 1.43 d5 0 0 0 -20 -19.14 0.86 d6 -4 -4.57 -0.57 -24 -20.00 4.00 d7 -8 -9.14 -1.14 -18 -19.14 -1.14 d8 -12 -10.00 2.00 -12 -10.00 2.00 d9 -6 -9.14 -3.14 -6 -9.14 -3.14 d 10 0 0 0 0 0 0 Associative: reaction intermediate is formed by including the incoming ligand in the coordination sphere and has higher coordination number than reactants or products • Lower coordination number complexes • Rates depend on the entering group SYSTE M Weak Field / High Spin Strong Field / Low Spin Oh OW CFAE Oh OW CFAE d0 0 0 0 0 0 0 d1 -4 -6.08 -2.08 -4 -6.08 -2.08 d2 -8 -8.68 -0.68 -8 -8.68 -0.68 d3 -12 -10.20 1.80 -12 -10.20 1.80 d4 -6 -8.79 -2.79 -16 -16.26 -0.26 D5 0 0 0 -20 -18.86 1.14 d6 -4 -6.08 -2.08 -24 -20.37 3.63 d7 -8 -8.68 -0.68 -18 -18.98 -0.98 d8 -12 -10.20 1.80 -12 -10.20 1.80 d9 -6 -8.79 -2.79 -6 -8.79 -2.79 d 10 0 0 0 0 0 0 It is a continuous single step process Two types exist Interchange associative (IA ) – Bond making more important Interchange dissociative (ID) – Bond breaking more important Ammine complexes of Co(III) are the most studied. Water is the medium of reaction. Usually replacement of NH3 derivatives is very slow, so only other ligands are considered. [Co(NH3)5X]2+ + H2O [Co(NH3)5(H2O)]3+ + X- Rate = k. [Co(NH3)5X]2+ . [H2O] Rate = k’. [Co(NH3)5X]2+ Charge on the complex Steric factors Effect of leaving group Effect of solvent Presence of pi-donors and acceptors as spectator ligands The increase in positive charge decreases the rate of reaction following a dissociative mechanism because the breaking the metal-ligand bond becomes difficult. For aquation of the Ru complexes the trend is as shown [RuCl6]3[RuCl3(H2O)3]0 1.0 s-1 2.1 x 10-6 s1 Complex [Co(NH3)5(NO3)]2+ [Co(NH3)5I]2+ [Co(NH3)5F]2+ Rate constant S-1 ~ 10-5 ~ 10-6 ~ 10-8 Thus it is proved that M-X bond breaking is very much important in aquation reactions than bond formation. The rate of aquation of [Co(NH3)5X]2+ depends on the stability of M-X bond. If the M-X bond is more stable rate of reaction is low. The order of reactivity is HCO3->NO3->I->Br->Cl->SO42-> F->SCN->NO2- This is the order of decreasing thermodynamic stability of the complexes formed with these groups Anation reactions do not depend very much on the nature of the entering group, Y-. Instead, it is very much dependent on the nature of the bond being broken. Experimental data show that the rate is of the order 10 -6 for the different entering groups (Y-), N3-, SO42-, Cl- or NCS- clearly indicating that the rate is independent of the nature of the entering group Another important experimental support for this observation is that ligand exchange reactions do not take place directly but instead takes place through aquation and then anation. [Co(NH3)5X]2++ Y [Co(NH3)5Y]2+ + X- This indicates that the Co-X bond breaking is very much significant and then whatever species is present at a higher concentration will add in anation reaction. Thus, nature of Y- When the non-leaving ligands are bulky, they will be crowding the central metal ion. The incoming ligand will find it difficult to approach the central metal ion slowing down the rate of reaction taking place by associative mechanism. Instead, if the reaction takes place by dissociative mechanism, the rate of the reaction will increase because the crowding around the metal ion is reduced. • Steric crowding around the metal centre favors dissociative activation • Dissociative activation relieves crowding around the complex • Steric crowding has been qualitatively and quantitatively explored – Tolman Cone Angle Complex k x 104 S-1 Complex Cis-[Co(NH3)4Cl2]+ Very fast [Co(NH3)5Cl]2+ (0) Cis-[Co(en)2Cl2]+ Cis-[Co(trien)Cl2]+ trans-[Co(NH3)4Cl2]+ trans-[Co(en)(NH3)2Cl2]+ trans-[Co(en)2Cl2]+ 150 90 1100 130 19 [Co(en)2(NH3)Cl]2+ (2) [Co(tren)(NH3)Cl]2+ (3) [Co(en)(dien) (NH3)Cl]2+(3) [Co(tetren)Cl]2+ (4) k x 104 S-1 4.0 0.85 0.40 0.31 0.15 AA k x 10 3 min -1 H2N-CH2-CH2-NH2 (en) 1.9 H2N-CH2-CH(CH3)-NH2 (pn) 3.7 H2N-CH(CH3)-CH(CH3)-NH2 (dl - bn) 8.8 H2N-CH(CH3)-CH(CH3)-NH2 (m – bn) 250 H2N-C(CH3)2-C(CH3)2-NH2 (tetrameen) instantaneous