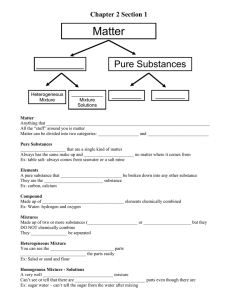
Describing and Classifying Matter UNIT 1.1 Guiding Questions (Activity 1.1) A. What is matter made of? B. What properties describe matter? C. How can you classify different types of matter? A. Matter and Its Nature Anything that has mass and takes up space is matter. It is composed of tiny particles called atoms. Chemistry is the study of matter and how it changes. Components of Matter Atom is the basic unit from which all matter is made. Elements substances that are made up of only one type of atom. A Molecule is a group of two or more atoms held together by chemical bonds. A Compound is a substance made of two or more elements that are chemically combined in a set ratio. Components of Matter Atom is the basic unit from which all matter is made. Elements substances that are made up of only one type of atom. A Molecule is a group of two or more atoms held together by chemical bonds. A Compound is a substance made of two or more elements that are chemically combined in a set ratio. B. The States of Matter Solid: a rigid substance with a definite shape Liquid: has a definite volume but takes the shape of its container Gas: takes the shape and volume of its container C. Classification of Matter C.1 Pure Substance Pure substances are substances that are made up of only one kind of particles and has a fixed or constant structure. Pure substances are further classified as elements and compounds. Characteristics of Pure Substance Pure substances are mostly homogeneous in nature containing only one type of atoms or molecules. These substances mainly have a constant or uniform composition throughout. The substances have fixed boiling and melting points. A pure substance usually participates in a chemical reaction to form predictable products. Examples of Pure Substance C.2 Mixture A mixture is impure if it consists of different kinds of elements combined together physically and not chemically. Mixtures are further divided into a homogenous or heterogeneous mixture. Types of Mixtures In a homogeneous mixture, it is difficult or impossible to see the different parts. In a heterogeneous mixture, the composition is not uniform throughout the mixture. The mixture of substances are not chemically combined to each other. Characteristics of Mixture It does not have any specific properties; the properties of the mixture are a result of the average properties of all the constituents. It is formed as a result of a physical change. They have a variable composition. Their melting and boiling points differ. Directions: Given the images below, identify if it is a pure substance or a mixture. Mixture Pure substance Mixture Pure substance Mixture METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 1. Hand Separation This method involves simply picking out all the unwanted substances by hand and separating them from useful ones, or maybe that both the separated substances are useful. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 2. Sieving It is done to separate mixtures that contain substances mostly of different sizes. The mixture is passed through the pores of the sieve. All the smaller substances pass through easily while the bigger components of the mixture are retained. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 3. Decantation Decanting means you are pouring off a liquid without disturbing the sediment or other liquid layers. It is usually used to separate solids from liquids, but it can be used to separate two liquids. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 4. Filtration The most common method of separating a liquid from an insoluble solid is the filtration. Take, for example, the mixture of sand and water. Filtration is used here to remove solid particles from the liquid. Various filtering agents are normally used like filtering paper or other materials. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 5. Magnetic Separation When one substance in the mixture has some magnetic properties then this method is quite useful. Strong magnets are commonly used to separate magnetic elements. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 6. Evaporation It is a process in which a liquid changes into gaseous form from heating. It allows the liquid to evaporate, leaving the soluble solid behind. METHODS IN SEPARATING THE COMPONENTS OF A MIXTURE 7. Distillation When mixtures consist of two or more pure liquids then distillation is used. Here the components of a liquid mixture are vaporized, condensed and then isolated. The mixture is heated and the component which is volatile vaporizes first. The vapor moves through a condenser and is collected in a liquid state. Directions: Directions: On the table below, identify the appropriate separation method for the mixtures provided. Mixture 1. Colored beads 2. Orange juice and its pulps 3. Paper clips and sand 4. Sugar and water 5. Soil and water Separation Method Review Questions Thank you!