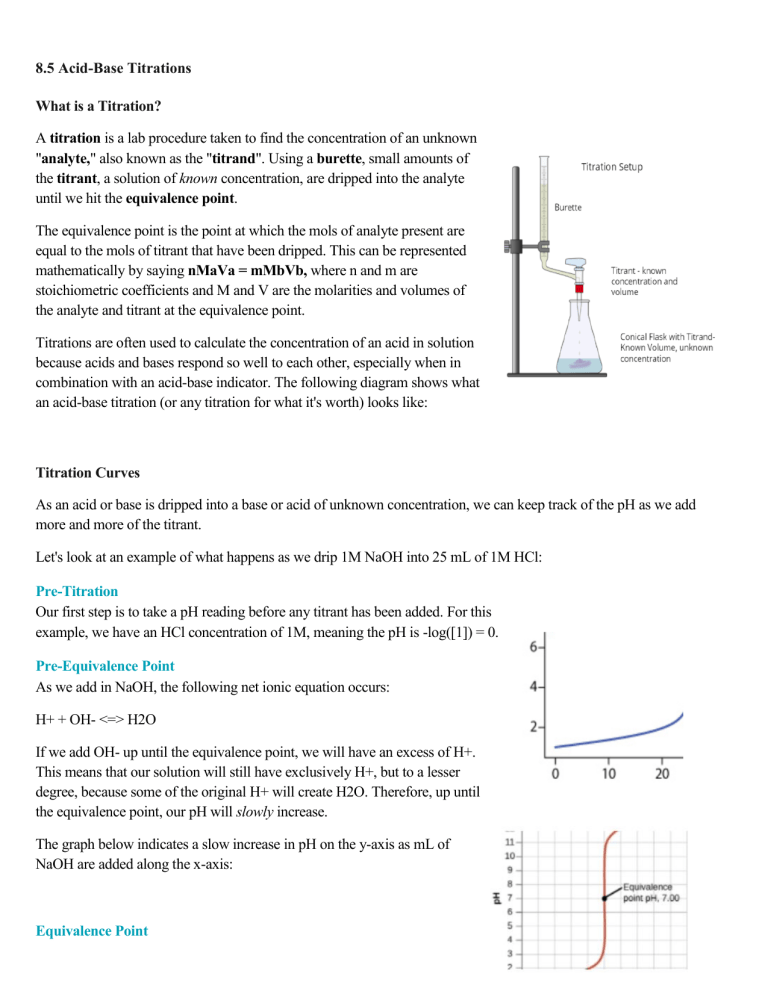
8.5 Acid-Base Titrations What is a Titration? A titration is a lab procedure taken to find the concentration of an unknown "analyte," also known as the "titrand". Using a burette, small amounts of the titrant, a solution of known concentration, are dripped into the analyte until we hit the equivalence point. The equivalence point is the point at which the mols of analyte present are equal to the mols of titrant that have been dripped. This can be represented mathematically by saying nMaVa = mMbVb, where n and m are stoichiometric coefficients and M and V are the molarities and volumes of the analyte and titrant at the equivalence point. Titrations are often used to calculate the concentration of an acid in solution because acids and bases respond so well to each other, especially when in combination with an acid-base indicator. The following diagram shows what an acid-base titration (or any titration for what it's worth) looks like: Titration Curves As an acid or base is dripped into a base or acid of unknown concentration, we can keep track of the pH as we add more and more of the titrant. Let's look at an example of what happens as we drip 1M NaOH into 25 mL of 1M HCl: Pre-Titration Our first step is to take a pH reading before any titrant has been added. For this example, we have an HCl concentration of 1M, meaning the pH is -log([1]) = 0. Pre-Equivalence Point As we add in NaOH, the following net ionic equation occurs: H+ + OH- <=> H2O If we add OH- up until the equivalence point, we will have an excess of H+. This means that our solution will still have exclusively H+, but to a lesser degree, because some of the original H+ will create H2O. Therefore, up until the equivalence point, our pH will slowly increase. The graph below indicates a slow increase in pH on the y-axis as mL of NaOH are added along the x-axis: Equivalence Point Then, we reach a turning point where the moles of HCl originally in the solution (25mmol) will equal the moles of NaOH added. This occurs at 25mL (we know this by solving the equation MaVa = MbVb). At 25mL, we have 25mmol of both HCl and NaOH, meaning we have no excess reactant and are left over with only water at the end. At the equivalence point, our pH is 7. This is true of any titration between a strong acid and a strong base. (We will get to weak acids and bases later). Post-Equivalence Point Once we reach past the equivalence point, we will have an excess of base, indicating that our pH slowly increases as we add more base, similarly to before the equivalence point. Overall, here is what the titration curve for this entire like: titration looks Titrations with Weak Acids and Bases The minor problem with our example above is that most acids and bases are not strong! However, the process is almost exactly the same when we have a strong acid and a weak base or a strong base and a weak acid (the latter is more common to see on the AP Exam). One subtle difference is that before you reach the equivalence point, you will have both acid and conjugate base (or base and conjugate acid) in the solution! What alarms should this be setting off? � BUFFER!!! � In the net ionic equation, we still have both the weak acid and the conjugate base because it does not fully dissociate. Take, for example, the titration of NaOH into acetic acid: CH3COOH + NaOH <=> CH3COONa + H2O CH3COOH + OH- <=> CH3COO- + H2O When we have an excess in CH3COOH, we end up with both CH3COOH and CH3COO-. A buffer! The same goes for the opposite situation (for example, the titration of HCl into NH4NO3). Thus, our solution is less responsive to changes in pH and will have a half-equivalence point at exactly 1/2 the volume of the equivalence point. At this point, we have the maximum buffer where pH=pKa or pOH=pKb (If you're titrating a weak acid with a strong base, use the former, and vice versa). Furthermore, because there is a product other than H2O, the pH at the equivalence point will not always be 7! In fact, it will never be 7. When titrating a weak base with a strong acid, your equivalence point will be acidic, and vice versa, a weak acid with a strong base will yield a basic equivalence point. This is because, at the equivalence point, you will create some conjugate base/acid. See an example of two titration curves, one with a strong base and weak acid and the other with a strong acid and weak base. Test yourself on whether you can find the half-equivalence point and equivalence point and estimate what the pH at the equivalence point is and the Ka/Kb of the acid/base! Reading a Burette Before we get to some examples, take a quick detour to learn how to read a burette. When doing a titration, this is an essential skill, as it allows you to see how much titrant you have added to the analyte. Let's go through an example FRQ: Example Problems Finding Molarity at the Equivalence Point Find the concentration of HF at the equivalence point when titrating HF with NaOH if the equivalence point occurs when 20mL of 0.1M NaOH is titrated into 10mL HF. For this problem, we can use our equation MaVa = MbVb, which describes how at the equivalence point we have equimolar quantities of titrant and analyte: Ma(10mL) = (0.1M)(20mL) Ma = (0.1)(20)/10 = 0.2M Therefore, the concentration of HF is 0.2M. Similar strategies can be used to find volumes at the equivalence point! Weak Acid/Strong Base Titration Find the pH of the solution formed from the titration of 25mL of 0.1M CH3COOH with 10mL 0.1M KOH (Ka = 1.8 * 10^-5) Let's start by writing out our reaction and finding our net ionic: CH3COOH + KOH <=> CH3COOK + H2O CH3COOH + OH- <=> CH3COO- + H2O Next, we find mmol and do the stoichiometry dance: 25 * 0.1 = 2.5mmol CH3COOH 10 * 0.1 = 1.0mmol OHCH3COOH + OH- <=> CH3COO- + H2O 2.5mmol 1.0mmol 0 1.5mmol 0mmol 1.0mmol 0 1.0mmol Finally, we can use the Henderson-Hasselbalch equation to find the pH: pH = pKa + log(1.0/1.5) = 4.74 + log(1/1.5) = 4.56 Weak Base/Strong Acid Titration The process with a weak base strong acid titration is essentially the same: Find the pH of the solution formed from the titration of 30mL of 0.5M NH3 with 10mL 0.1M HCl (Kb = 1.8 * 10^-5) First, let's write out our net ionic: NH3 + HCl <=> NH4Cl NH3 + H+ <=> NH4+ Next, we find mmols and do stoichiometry: 30mL * 0.5M = 15mmol NH3 10mL * 0.1M = 1mmol HCl NH3 + H+ <=> NH4+ 15mmol 1mmol 0mmol 14mmol 0mmol 1mmol Finally, use the Henderson-Hasselbalch for bases to find the pOH: pOH = pKb + log(1/14) = 4.74 + log(1/14) = 3.59 Subtract from 14 to find that pH = 10.41 8.6 Molecular Structures of Acids and Bases Describing Acid and Base Strength The following big idea is all about looking at the molecular structure (think Lewis diagrams) of acids and bases to determine their strength. Previously, we learned that there are strong acids/bases and weak acids/bases. How can we differentiate these visually? The first step in using molecular structures to draw conclusions about strength is understanding how we can describe acids and bases as strong/weak based on the molecules themselves. Essentially, strength stems from the stability of the conjugate base/acid of the acid/base in question. Remember that when describing the strength of a conjugate acid/base, it is inversely proportional to the strength of the base/acid itself. That is to say that the stronger the acid, the weaker the conjugate base. If our conjugate base is a very strong base, our acid is not very strong, and vice versa. To know if a conjugate base is very strong, we discuss the stability of the compound. The question we want to answer for conjugate bases is 'will this compound attract an H+ ion?" For conjugate acids, 'will this compound readily donate an H+ ion?' Connecting Strength to Structure Because we now understand how the strength of a conjugate base/acid relates to the strength of its parent compound, we are ready to move into looking at different structures. One of the first ideas is that weaker bonds to an acidic H will lead to a stronger acid because that bond is more easily broken. For example, in halogenic hydrides (eg. HF, HI, HBr, HCl), the weakest H-X interactions occur with larger halogens. Therefore, moving down the halogen, the acids become stronger because larger atoms will give weaker interactions. Similarly, acid strength increases from left to right across a period and increases going down a group. This can actually help us understand why strong acids have very weak conjugate bases. When a strong acid, like HCl, dissociates, the conjugate base, Cl-, is incredibly stable. Cl- is a stable ion that will not readily react with much. Ultimately, it is not a reactive base and will create a more dissociative acid. When discussing oxyacids, the structure can be described by looking at the polarity of the bond between the acidic oxygen (the oxygen attached to the acidic hydrogen). The easier it is for that O-H bond to break, the stronger the acid. In the following image, we represent the "rest" of the acid as "Z." The OH bond is easy to break when Z is either very electronegative or has a high oxidation state. An example of a class of weak acids that are oxyacids is carboxylic acids. Carboxylic acids have a COOH group on the end, such as CH3COOH. Because of the low electronegativity on the carbon, making the bond less polar, these acids are relatively weak. 8.7- pH and pKa There are many measures in AP Chemistry, especially relating to acids and bases and equilibrium. In this section, we will discuss the relationships between pH and pKa, two of the most important measures! ‘p’ Notation 'p' notation is actually fairly simple and seen throughout acid-base chemistry (we have pH, pOH, pKa, pKb, and many more!). 'p'something is simply equal to the -log(something). For example, pH = -log(H+), and pOH = -log(OH-). Similarly, pKa = -log(Ka). pKa and Acid Strength An important use of pKa is in describing acid strength relative to other acids. For example, if one acid has a pKa of 3 and another has a pKa of 2, we know that the acid with a pKa of 2 is 10 times as acidic (note, however, that this does not mean that the pH is 10 times lower). Using 'p' notation gives us a logarithmic scale and not a linear one. Like pH, where a lower pH corresponds to a higher [H+], a lower pKa implies a higher Ka. However, it is worth noting that a high pKa does not imply basicity. Another note is that, like pH and pOH, pKa + pKb = 14. pH, pKa, and Buffers pH and pKa are also related to buffers. As a reminder, a buffer is a mixture of an acid and its conjugate base and is important because it is resistant to changes in pH. However, a question arises: when is the buffer the strongest? The Henderson-Hasselbalch Equation can be applied to find the pH of a buffer: The strongest buffer occurs when the concentration of [A-] is equal to [HA]. In this case, pH = pKa + log(1) ⇒ pH = pKa. The relationship is vital, especially when looking at titration curves, because this same point occurs at the half-equivalence point, implying that you have the optimal buffer at the half-equivalence point. Acid-Base Indicators Finally, we will discuss acid-base indicators. Acid-base indicators are a class of compounds that change color depending on the pH of the solution they are in. You may have used indicators in class during titrations to note when the equivalence point of a titration occurs. Some examples of acid-base indicators are bromothymol blue, phenolphthalein, and methyl red. When choosing an acid-base indicator, you usually want to pick one in which your pH will end up in the effective range, which is the pKa plus or minus 1. While you will not need to memorize any indicators or their effective ranges on the exam, you may be asked to pick which one is the most effective for a certain experiment. Let's see this concept with an FRQ from 2010: We are given the following prompt: In order for the indicator to be useful to us, we want it to change color at the equivalence point for this titration. Looking at the graph, we see that the pH at the equivalence point is 7. We also know this because it is a strong acid strong base titration. Therefore, we want to pick an indicator with a pH range closest to 7. This turns out to be methyl red, which is the correct answ 8.8- Properties of Buffer Buffers Review As we mentioned, buffers are special solutions that are resistant to pH changes when adding acids or bases to them. Buffers are formed in a very specific way: creating a solution of a weak acid and its conjugate base (or a weak base and its conjugate acid, but the former is much more common). It is important that the acid you create a buffer with is weak because otherwise, the conjugate base would not be a significant base. For example, a mixture of HCl and NaCl would not be a buffer despite being a combination of an acid (HCl) and its conjugate base (Cl-). You may be asking then why any weak acid isn't a buffer. At equilibrium, there is so much more acid than the conjugate base (assuming a low Ka) that the buffer effects are negligible. In order for a buffer to be effective, you must have comparable concentrations of acid and conjugate base. In fact, the maximum buffer, the point at which the buffer most effectively resists pH change, occurs when the concentration of acid is equal to the concentration of the conjugate base. Solidify this concept by doing a few practice problems. For each of the pairs of compounds given, identify them as a pair that would form a buffer or not form a buffer: NaOH and Na+: o CH3COOH and Ca(CH₃COO)₂: o This pair does form a buffer. NH3 is a weak base, and NH4+ is a significant acid (and its conjugate acid), meaning this pair forms a buffer. In this case, like Ca2+ in the previous example, the nitrate ion is simply a spectator. HI and I: o The answer to this question is yes! When dissolved together, this pair will form a buffer. CH3COOH is a weak acid (acetic acid AKA vinegar) with a Ka=1.8 * 10^(-5). Ca(CH3COO)2 is calcium acetate, which will dissociate into Ca2+ (a spectator ion as far as the buffer is concerned), and two moles of CH3COO-, the conjugate base of CH3COOH! Because acetic acid is a weak acid, CH3COO- is a significant base, meaning that we will have a buffer. NH3 and NH4NO3: o The answer to this question is no. Although NaOH and Na+ are a base-conjugate acid pair, remember that NaOH is a strong base. This means that Na+ is not a significant acid and will not form a buffer. Like example one, this pair does not form a buffer. HI is a strong acid and cannot form buffers with its conjugate base I- because I- is not a significant base. KI and PbNO3: o It should be pretty easy to see that this pair does not form a buffer. There are no acids or bases involved. In fact, when you mix KI and PbNO3, you get the "golden rain" reaction, a precipitation reaction that forms PbI2 and KNO3. Take a look! What Makes Buffers Cool: pH Resistance Why do buffers have pH resistance, and what makes them so interesting and useful to study? Buffers have pH resistance because of the presence of an acid and a base that do not actively react together at equilibrium. This graphic shows what happens when an acid or a base is added to a buffer: When a strong acid is added to a buffer, the conjugate base eats it up and forms HAn (An = anion). In the case of no buffer, the strong acid would completely dissociate into H+, increasing [H+] to a much higher degree. Similarly, if OH- from a strong base is added to a buffer, the HAn present in the solution reacts with it to form An- and H2O instead of letting it produce pure OH-. These two reactions lead to buffers being resistant to pH! 8.9- Introduction to the Henderson-Hasselbalch Equation This section focuses intimately on one equation: the Henderson-Hasselbalch equation. The Henderson-Hasselbalch equation is useful because it helps us find the pH of a buffer. Thinking back to 8.8, a buffer is a solution that resists changes to its pH and is composed of a weak acid and its conjugate base or a weak base and its conjugate acid. Let's take a look at the Henderson-Hasselbalch equation and get ourselves situated with it: Breaking Down The Equation Breaking the equation down, pH is the -log([H+]) and is oftentimes the unknown when we apply the Henderson-Hasselbalch Equation. pKa is -log(Ka) is a logarithmic scale to describe the acidity of an acid (lower pKa = more acidic). Finally, we get to the new bit, the log base 10 of the ratio of the concentrations of an ion, A-, and an acid HA. We can start by realizing that there is a unique relationship between [A-] and [HA]. They constitute a conjugate acid-base pair! HA is a weak acid, and A- is its conjugate base! This is where the Henderson-Hasselbalch equation ties into buffers because you will always have a concentration of conjugate base and a concentration of conjugate acid. It also shows why the strongest buffer is when these concentrations are equal because then log([A-]/[HA]) = 0. Example Problems Example Problem #1: Directly Stated Buffer Find the pH of a buffer with 0.5M CH3COOH mixed with 0.25M CH3COONa (Ka = 1.8 * 10^-5). For this, we can plug directly into the Henderson-Hasselbalch: Example Problem #2: Using the Hasselbalch During A Titration Calculate the pH in the titration of 25.0 mL of 0.100M acetic acid with 0.100M NaOH after adding 15.0 mL of 0.100M NaOH. Start by writing out our net ionic equation for this reaction: CH3COOH + NaOH <=> CH3COONa + H2O CH3COOH + OH- <=> CH3COO- + H2O Next, we can use stoichiometry to find how many mmol of each compound we have after the reaction goes forward: CH3COOH + OH- <=> CH3COO- + H2O Start: 2.5mmol CH3COOH, 1.5mmol OH-, 0mmol CH3COO-, 0mmol H2O End: 2mmol CH3COOH, 0mmol OH-, 1.5mmol CH3COO-, 1.5mmol H2O Because we have concentrations of both an acid and its conjugate base, we can find the pH of this by finding the pH of that buffer using the Henderson-Hasselbalch. Note that because we are dividing by the same volume to find concentration, they cancel out and we can just divide the mmols: 8.10 Buffer Capacity For a quick reminder, buffers are important because they are resistant to changes in pH. However, buffers are not infinitely resistant. Eventually, the buffer will weaken and succumb to the acid/base being added. This is why, despite there being buffers in your bloodstream, chugging hydrochloric acid is a very bad idea. Seriously, don't do it! Buffer capacity helps us see how much acid/base one can add until there is a significant change in pH. Describing Buffer Capacity As said by the Henderson-Hasselbalch equation, the pH of a buffer is defined by the ratio of the concentrations of the conjugate base to the acid, or in math terms [A-]/[HA]. The capacity of a buffer is determined by the magnitudes of these concentrations. What do we mean when we refer to magnitude? Essentially, the magnitude of each concentration describes how large the concentrations are. A concentration of 5M would have a higher magnitude than a 0.5M solution. The more concentrated the acid and conjugate base, the stronger the buffer is at reducing pH changes! There is more acid and conjugate base to be resistant to strong acids/strong bases in a similar volume. Example Problem: Identifying the Stronger Buffer To practice applying buffer capacity, try thinking about two separate buffer systems, one with 5M acetic acid and 5M sodium acetate and another with 0.05M acetic acid and 0.05M sodium acetate. Because the ratios of the conjugate base to the acid are the same, both of these buffers will have the same pH (pH=4.74). However, our question asks us the following: After HCl is added to each buffer system, the first one has a resulting pH of 4.74, and the second one has a resulting pH of 4.56. Which one has the better buffering capacity and why? We can see that in the first buffer, the pH remained relatively unchanged, whereas, in the second, the pH dropped. Because the first system's pH remained constant, the first buffer is more effective at resisting pH changes and, therefore, has a better buffering capacity. The magnitude of the concentration of both the acid and the conjugate base is higher in the first buffer compared to the second, implying the first buffer will also have a stronger buffer capacity. Practice Multiple Choice Questions The College Board likes to use buffer capacity specifically on multiple choice questions because, unlike many other questions relating to buffers, questions about buffer capacity are very often qualitative. That means your answer will relate to some sort of non-numerical conclusion based on the information you are given. They could ask you to identify the stronger buffer, like in the last question, or ask about a change in a system and how this may affect the buffer capacity. Here is another example: This problem gives us a BUNCH of information and can be really overwhelming at first. However, by breaking it down piece by piece, the problem is more approachable. We know that a student is creating a buffer of acetic acid (CH3COOH, also stated as HC2H3O2) and sodium acetate since a weak acid mixes with its conjugate base. Now we can examine the numbers. The first set of numeric information indicates that the student wants to mix 250 mL of 0.100M acetic acid with 500 mL of 0.440M sodium acetate. Yikes! The student makes an error. They choose an acid with half the concentration and a conjugate base with half of the volume. For acetic acid, the volume remains at 250mL, but the molarity is now 0.0500M. Meanwhile, the molarity of the sodium acetate stays at 0.440M, but the volume falls from 500mL to 250mL. The problem wants to know the ramifications of these mistakes. Before looking at the answer choices, what changed? In both sets (the weak acid and the conjugate base), the number of moles is halved. Therefore, we will have half the number of moles of each species in our buffer. Again, before looking at any answer choices, how is the buffer impacted? As we discussed earlier, a lower number of moles of each reactant in the buffer creates less resistance to changes in pH. In other words, the buffer capacity lowers. Which answer choice matches that concept? If we read the answer choices, we see that what we just described fits answer A. You may be tempted to pick B, but remember that buffer capacity is determined by the number of moles of the weak acid and the conjugate base.