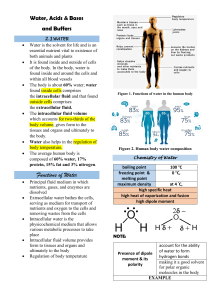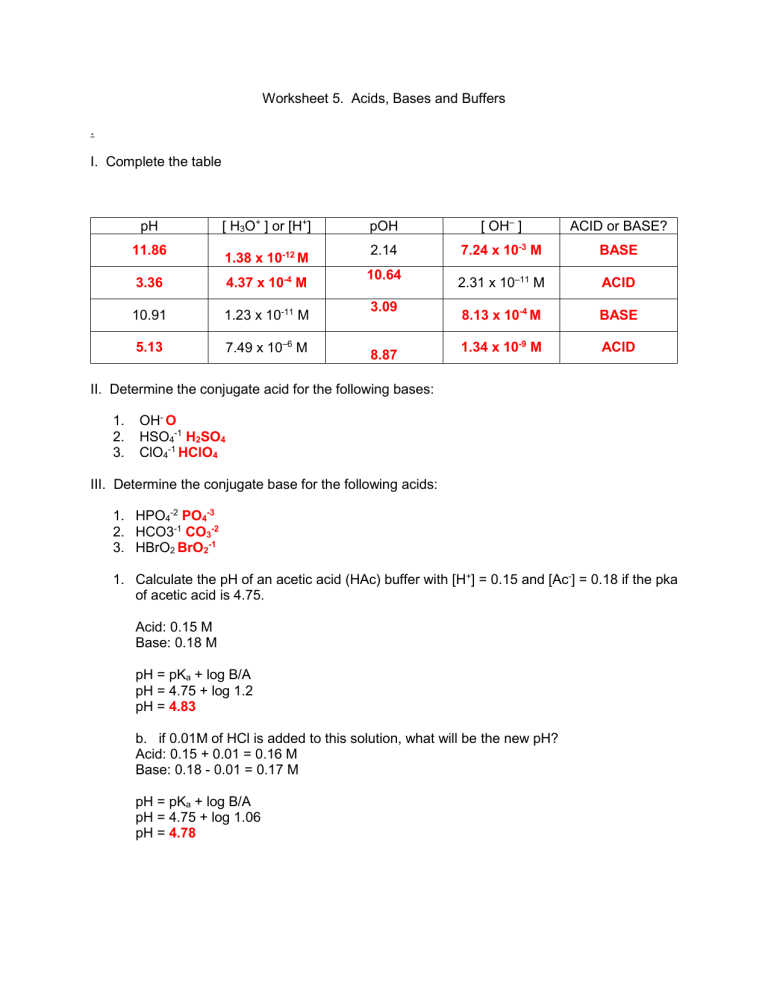
Worksheet 5. Acids, Bases and Buffers . I. Complete the table pH [ H3O+ ] or [H+] pOH [ OH– ] ACID or BASE? 11.86 1.38 x 10-12 M 2.14 7.24 x 10-3 M BASE 3.36 4.37 x 10-4 M 10.64 2.31 x 10–11 M ACID 10.91 1.23 x 10-11 M 3.09 8.13 x 10-4 M BASE 5.13 7.49 x 10–6 M 1.34 x 10-9 M ACID 8.87 II. Determine the conjugate acid for the following bases: 1. 2. 3. OH- O HSO4-1 H2SO4 ClO4-1 HClO4 III. Determine the conjugate base for the following acids: 1. HPO4-2 PO4-3 2. HCO3-1 CO3-2 3. HBrO2 BrO2-1 1. Calculate the pH of an acetic acid (HAc) buffer with [H+] = 0.15 and [Ac-] = 0.18 if the pka of acetic acid is 4.75. Acid: 0.15 M Base: 0.18 M pH = pKa + log B/A pH = 4.75 + log 1.2 pH = 4.83 b. if 0.01M of HCl is added to this solution, what will be the new pH? Acid: 0.15 + 0.01 = 0.16 M Base: 0.18 - 0.01 = 0.17 M pH = pKa + log B/A pH = 4.75 + log 1.06 pH = 4.78 c. if 0.01M of NaOH is added to this solution, what will be the new pH? Acid: 0.15 - 0.01 = 0.14 M Base: 0.18 + 0.01 = 0.19 M pH = pKa + log B/A pH = 4.75 + log 1.36 pH = 4.88 d. Is this a good buffer? Justify your answer. Buffer Range: 4.83-1 = 3.83 4.83+1= 5.83 Yes, it is a good buffer because it is in the buffer range.