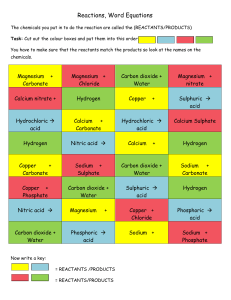
Question1 Complete the following word equations 1- Calcium carbonate + hydrochloric acid calcium chloride + water + __________________ 2 ___________________________ + sulfuric acid magnesium sulfate + hydrogen 3. Magnesium + nitric acid ___________________________ + Hydrogen 4. Zinc hydroxide + ___________________________ zinc chloride + water 5. ____________________ + hydrochloric acid magnesium chloride + water + carbon dioxide 6. Aluminium + hydrochloric acid ________________ + hydrogen 7. sodium hydroxide + hydrochloric acid ________________ + ________________ 8. sodium carbonate + _________________ sodium sulfate + water + ________________ 9.___________________________ + nitric acid calcium nitrate + water + carbon dioxide 10. ___________________________ + sulfuric acid iron(II) sulfate + hydrogen Khalid A Hameed Page 1 Question2 Complete the following word equations 1- Hydrochloric acid + sodium hydroxide ………………………………. + …………………………………… 2- Sodium + water ………………………………………. + …………………………………. 3- Magnesium + oxygen ……………………………………………………………………… 4- Sulfuric acid + magnesium carbonate …………………………….. + …………………………….. + …………………………………………. 5- Zinc + copper sulfate ………………………………… + ……………………………………… 6- Sulfuric acid + sodium hydroxide .....…………………………. + …………………………………….. 7- Hydrogen + oxygen …………………………………… 8- Carbon + oxygen ………………………………………… 9- Aluminuim + nitric acid ………………………………………. + ……………………………… 10- Sodium + sulfur Khalid A Hameed ……………………………………………………………………….......... Page 2 Question 3 Complete and balance the symbol equations (you are allowed to write in the spaces only) 1. __________________ + 2HCl(aq) MgCl2(aq) + H2O(l) + ______________ 2. Mg(OH)2(s) + H2SO4(aq) _______________ + __________________ 3. CuO(s) + __________________ Cu(NO3)2(aq) + H2O(l) 4. ________________ + H2SO4(aq) MgSO4(aq) + H2O(l) 5. __________________ + H2SO4(aq) MgSO4(aq) + H2O(l) + CO2(g) 6. Zn(OH)2(s) + 2HCl(aq) ZnCl2(aq) + __________________ 7. Mg(s) + H2SO4(aq) ?__________________ +__________________ Khalid A Hameed Page 3 Question 4 Complete the following word equations and write balanced chemical equations f 1- Calcium carbonate + hydrochloric acid …………………………………………………………………………………………………………………… …………………………………………………………………………………………………………………… 2- Iron (III) oxide + sulfuric acid …………………………………………………………………………………………………………………… ……………............................................................................................................ 3- Sodium carbonate + sulfuric acid …………………………………………………………………………………………………………………… ……………............................................................................................................ 4- Zinc + hydrochloric acid …………………………………………………………………………………………………………………… ……………............................................................................................................ 5- Aluminium + nitric acid …………………………………………………………………………………………………………………… ……………............................................................................................................ Khalid A Hameed Page 4 Question 5 Read the following paragraphs about some chemical processes (reactants and products are underlined): In car engines, where the temperature is very high, nitrogen gas reacts with oxygen to form different oxides, one of these oxides is nitrogen dioxide which contributes to acid rain (Reaction 1). Carbon monoxide, a poisonous gas, is also formed by the reaction of oxygen and carbon (present in fuels). (Reaction2). Car manufactures use catalytic convertors to change these gases into the less harmful products nitrogen, oxygen and carbon dioxide. During the process of photosynthesis, plant combine carbon dioxide gas from the atmosphere and water from the soil to produce glucose (C6H12O6) and oxygen gas (Reaction 3). In respiring, some living organisms burn glucose to produce carbon dioxide gas and water vapor. (Reaction4). In an industrial process, limestone (calcium carbonate) is strongly heated to produce quicklime (calcium oxide) and carbon dioxide gas (reaction 5). In the presence of water, iron (and steel) articles react with oxygen in the air to form iron (III) oxide (Reaction 6). In industry, zinc metal is extracted from zinc blend (zinc sulfide) by heating the blend in air. The products are sulfur dioxide and zinc oxide (reaction7) The metal oxide is then heated with carbon to produce the pure metal and a colourless gas (reaction 8) Write word and balanced chemical equations for reactions 1 to8 Khalid A Hameed Page 5 Reaction 1: ………………………………………………………………………………………. .………………………………………………………………………………….. (3) Reaction 2: ……………………………………………………………………………………… ………………………………………………………………………………….. (3) Reaction 3: ……………………………………………………………………………………… ………………………………………………………………................................ (3) Reaction 4: ……………………………………………………………………………………… ………..………………..…………………………………………………………(3) Reaction 5: ………………………………………………………………………………………. .…………………………………………………………………………………. (3) Reaction 6: ……………………………………………………………………………………… …………………………………………………………………………………… (3) Reaction 7: ……………………………………………………………………………………… …………………………………………………………………………………… (3) Reaction 8: ……………………………………………………………………………………… …………………………………………………………………………………… (3) [Total for Q 5, 24marks] Khalid A Hameed Page 6 Khalid A Hameed Page 7