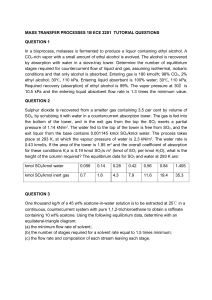
5. In the reaction of oxygen atoms with benzene the following data was found experimentally: k(M-1s-1) T(K) 1.44 x 107 300 3.03 x 107 341 6.9 x 107 392 a) What is the order of this reaction and how do you know that? b) Calculate the activation energy and the Arrhenius preexponential factor. You do not need to plot the data. c) Estimate the reactive cross section at 340K d) Estimate the steric effect , p. Take the radii to be r(O) = 78pm and r(benzene) = 265pm (1pm = 10-12m) and m(O) = 16amu and m(benzene) = 78amu. Arrhenius equation: k = Ae-Ea/RT Collision Theory rate of reaction = f x collision density 1 8k T 2 n collision frequency z = b N A (per molecule) V 1 8k T 2 n n collision density ZAB = b A B N A2 V V f = fraction of successful collisions = e E NA o RT (per molecule) where EoNA = Ea Activated Complex Theory k T RT 2 e e k= b h P S R e Ea RT k T RT RS RTH e e = b h P Constants Atomic mass unit, mu = 1.66 x 10-27 kg h = 6.626 x 10-34 Js kb = 1.380 x 10-23 J/K NA = 6.022 x 1023/mol G = H – TS J = kgm2/s2 R = 8.314 J/Kmol 1.987cal/Kmol 0.08206 atmL/Kmol 0.08314barL/Kmol k 14400000 30300000 69000000 Ea A T 300 341 392 ln k 16.48 17.227 18.050 16641.30 slope*R 11164444865.0 exp(23.136) 1/T 0.00333 0.00293 0.00255