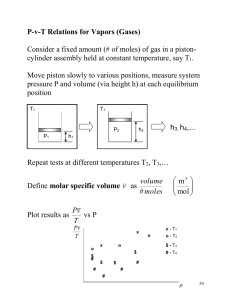
5-22 gas is accelerated in a nozzle to 450 m/s. The... 5-37
advertisement
5-22 5-37 CO2 gas is accelerated in a nozzle to 450 m/s. The inlet velocity and the exit temperature are to be determined. Assumptions 1 This is a steady-flow process since there is no change with time. 2 CO2 is an ideal gas with variable specific heats. 3 Potential energy changes are negligible. 4 The device is adiabatic and thus heat transfer is negligible. 5 There are no work interactions. Properties The gas constant and molar mass of CO2 are 0.1889 kPa.m3/kg.K and 44 kg/kmol (Table A-1). The enthalpy of CO2 at 500C is h1 30,797 kJ/kmol (Table A-20). 1 m 2 m . Using the ideal gas relation, the specific volume is Analysis (a) There is only one inlet and one exit, and thus m determined to be v1 RT1 0.1889 kPa m 3 /kg K 773 K 0.146 m 3 /kg P1 1000 kPa 1 CO2 2 Thus, m 1 v1 A1V1 V1 m v1 6000/3600 kg/s 0.146 m3 /kg 60.8 m/s A1 40 10 4 m 2 (b) We take nozzle as the system, which is a control volume since mass crosses the boundary. The energy balance for this steady-flow system can be expressed in the rate form as E E out in Rate of net energy transfer by heat, work, and mass E system0 (steady) 0 Rate of change in internal, kinetic, potential, etc. energies E in E out pe 0) m (h1 V12 / 2) m (h2 + V22 /2) (since Q W 0 h2 h1 V 22 V12 2 Substituting, h2 h1 V 22 V12 M 2 30,797 kJ/kmol 450 m/s2 60.8 m/s2 2 1 kJ/kg 1000 m 2 /s 2 44 kg/kmol 26,423 kJ/kmol Then the exit temperature of CO2 from Table A-20 is obtained to be T2 = 685.8 K PROPRIETARY MATERIAL. © 2011 The McGraw-Hill Companies, Inc. Limited distribution permitted only to teachers and educators for course preparation. If you are a student using this Manual, you are using it without permission.