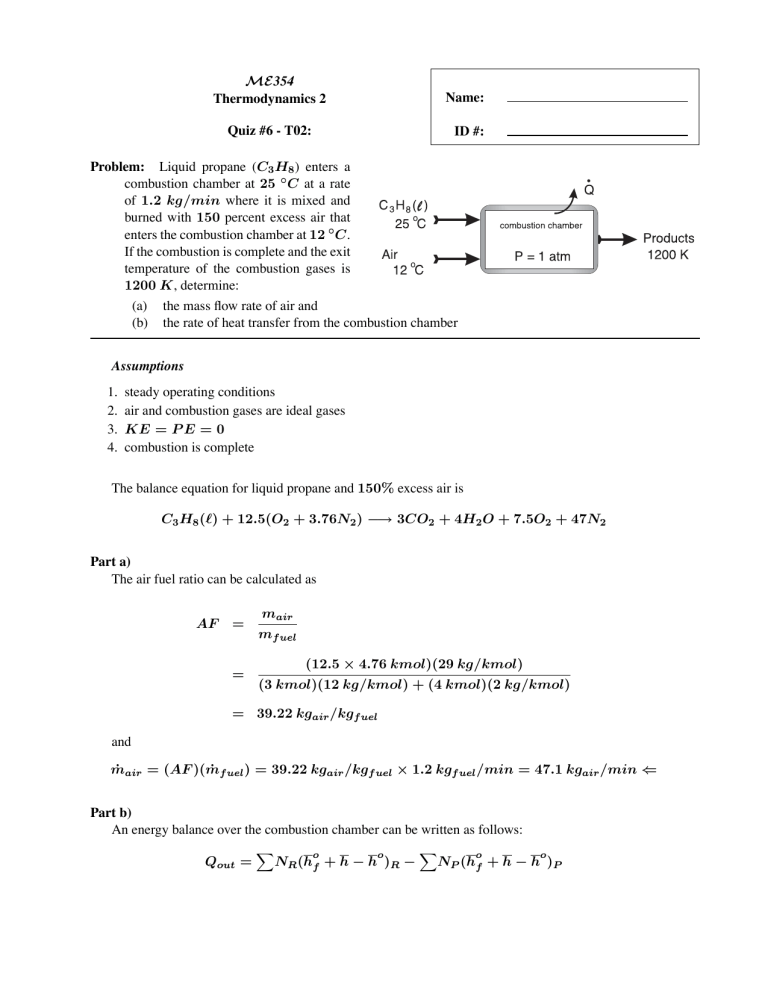
ME354 Thermodynamics 2 Name: Quiz #6 - T02: ID #: Problem: Liquid propane (C3 H8 ) enters a combustion chamber at 25 ◦ C at a rate of 1.2 kg/min where it is mixed and burned with 150 percent excess air that enters the combustion chamber at 12 ◦ C. If the combustion is complete and the exit temperature of the combustion gases is 1200 K, determine: (a) the mass flow rate of air and (b) the rate of heat transfer from the combustion chamber Assumptions 1. 2. 3. 4. steady operating conditions air and combustion gases are ideal gases KE = P E = 0 combustion is complete The balance equation for liquid propane and 150% excess air is C3 H8 () + 12.5(O2 + 3.76N2 ) −→ 3CO2 + 4H2 O + 7.5O2 + 47N2 Part a) The air fuel ratio can be calculated as AF = = mair mf uel (12.5 × 4.76 kmol)(29 kg/kmol) (3 kmol)(12 kg/kmol) + (4 kmol)(2 kg/kmol) = 39.22 kgair /kgf uel and ṁair = (AF )(ṁf uel ) = 39.22 kgair /kgf uel × 1.2 kgf uel /min = 47.1 kgair /min ⇐ Part b) An energy balance over the combustion chamber can be written as follows: Qout = o o NR (hf + h − h )R − o o NP (hf + h − h )P o Substance hf (kJ/kmol) C3 H8 () O2 N2 H2 O(g) CO2 -118,623 0 0 -241,820 -393,520 h285 K (kJ/kmol) h298 K (kJ/kmol) h1200 K (kJ/kmol) 8,296.5 8,286.5 8682 8669 9904 9364 38,447 36,777 44,380 53,848 where we note from Tables A-26 and A-27 o o hf () = hf (g) − hf g = −103, 850 kJ/kmol − (44.097 kg/kmol × 335 kJ/kg) = −118, 623 kJ/kmol Substituting we get Qout = (1)(−118, 623) + (12.5)(0 + 8296.5 − 8682) + (47)(0 + 8286.5 − 8669) −(3)(−393, 520 + 53, 848 − 9364) − (4)(−241, 820 + 44, 380 − 9904) −(7.5)(0 + 38, 447 − 8682) − (47)(0 + 36, 777 − 8669) = 190, 751.25 kJ/kmolC3 H8 The rate of heat transfer is Q̇ = Ṅ Qout = ṁ N Qout = = 5, 202.3 kJ/min ⇐ 1.2 kg/min 44 kg/kmol (190, 751.25 kJ/kmolC3 H8 )








