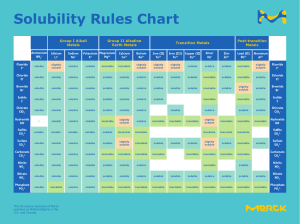
• • • • • • • • Contents Introduction Classification of Monophasic liquid Formulation Consideration Manufacturing Consideration Recent advance in monophasic liquid Recent approach in monophasic liquid formulation Conclusion Recommendation Introduction • Monophasic dosage form refers to liquid preparation containing two or more components in one phase system, it is represent by true solution. • A true solution is a clear homogenous mixture that is prepared by dissolving solute in a suitable solvent. • The component of the solution which is present in a large quantity is known as “SOLVENT” where as the component present in small quantity is termed as “SOLUTE”. Advantage • It is easier to swallow, therefore easier for children and old age people. • Facilitate absorption of drug faster than solid dosage form as drug is already in solution form. • It is homogenous therefore give uniform dose than suspension or emulsion which need shaking. • Simple and fast to formulate • It can be administered by various routes : Oral, Parenteral (injection),enema for rectal use, otic(ear), nasal and ophthalmic preparation. Disadvantage • They are bulky, so difficult to transport and store. • Water is commonly use vehicle, which is prone to microbial growth. So addition of preservative is needed. • When expose to direct sunlight it may undergo hydrolysis, so need to store in cool and dark place. • Drug stability reduce by hydrolysis or oxidation. So, they have shorter expire date than solid dosage form. • Other major sign of drug instability are color change, Precipitation, microbial growth etc. Classification Liquids meant for internal administrations • Syrup : Aqueous preparations of 60% to 85% sucrose with or without flavoring agents and medicinal substances. e.g. Chlorpheniramine maleate syrup, Chloral hydrate . • Elixirs : Clear, aromatic, sweetened hydro alcoholic solutions with or without medicinal substances, intended for oral use. Eg: Dexamethasone elixir . • Linctuses : Viscous, liquid and oral preparations that are generally prescribed for the relief of cough. Eg: Codeine Linctus. Market available Syrup/Elixir/ Linctus Liquids meant for external administration Liquids used in the mouth • Gargles :Aqueous solutions containing antiseptics or antibiotics used to treat throat infections. Available in concentrated form with direction for dilution with warm water before use. eg: Povidone Iodine gargle. • Mouthwash: Aqueous solution with a pleasant taste and odor used to clean and deodorize the buccal cavity. Have antiseptic and astringent activity.eg: Antiseptics-phenol derivatives. • Throat paints : Viscous liquid preparation used for mouth and throat infections. Eg: Phenol glycerine, Compound Iodine. Market available Gargle, Mouthwash and Throat paints Liquids meant for external administration Liquids instill into body cavity • Eye drops: Sterile, aqueous/oily solutions intended for instillation in eye. Eg: Timolol maleate eye drops. • Nasal drops Administered through the nose to obtain local effect. Used during nasal congestion and upper-respiratory tract problem. Eg: Oxymetazolin Hydrochloride nasal drops. • Enemas: Aqueous or oily solution that is introduced into the rectum and colon via the anus for cleansing, therapeutic or diagnostic purposes. Market available Eye drop, Nasal drop and Enemas Liquids meant for external administration Liquid meant for skin • Liniments : Oily liquid preparations, intended for external application with rubbing action to the affected area. Use to relief pain and stiffness, such as from muscles spasm and arthritis. • Lotions : Topical preparation with a low to medium viscosity. Use to moisturize dry skin. Eg: Calamine Lotion, baby lotion • Paints : Solutions used to sterilize the skin. Eg. Betadine antiseptic paint, Magenta paint Market available Liniment, Lotion and Paint Consideration Formulation Consideration Manufacturing Consideration • Solubility • Raw Materials • Stability • Preservatives • Pharmaceutical elegance Viscosity modifiers Sweetening agents Flavouring agents Colouring agents • Equipments • Manufacturing Procedure Approaches to increase the solubility of the drug pH adjustment : By addition of buffer to the formulation . Co-solvency: By addition of water miscible solvent in which drug has good solubility. The solvent known as co-solvent. Micelle solubilization : At high concentration surfactant s are forced into water to form colloidal aggregate known as micelle. Drugs get adsorbed into micelle that increase drug solubility. Micelle form only at critical micelle concentration. Complexation: Drug-complexing agent complexation formed when complexing agent is added to solution. It increase solubility of drug on the basis of Le Chatelier’s principle or “ The equilibrium law”. Micronization: The process involve size reduction of drug particle 1 to 10microns either by spray drying or fluid energy mill. Hydrotrophy : Drug dissolve in the cluster of hydrotropic agent. Also there is drug- hydrotrophy agent complexation formation to increase drug solubility. Preservative Preservatives must have following criteria: • Effective against broad spectrum of microorganisms. • Physically, chemicaly and microbiologically stable for lifetime of the product. • Non toxic, non sensitizing, soluble, compatible and with acceptable taste and odour. Types of Preservatives • Acidic : phenol, benzoic acid, sorbic acid • Neutral preservatives : chlorobutanol, benzyl alcohol • Quarternary ammonium compounds : Benzalkonium chloride Stability Chemical Stability Physical Stability A stable formulation retains its Chemical stability of a formulation viscosity, color, clarity, taste, is affected by: and odour throughout its shelf pH life Temperature Ionic Strength Objective Solvent effects Subjective evaluation evaluation Light Colour can be measured Taste and odour can be Oxygen spectrophotometrically. determined either by pharmaceutical Instability can be prevented by Clarity can be determined by investigator or by a panel use of: measurement of its of unbiased, taste sensitive o Buffering agents turbidity or light individuals. o Antioxidants scattering equipment. o Proper packaging (eg: use of amber bottle for light Viscosity can be measured by use of sensitive products) viscometers. Pharmaceutical Elegance Viscosity modifiers Sweetening agents Enhance viscosity. Eg: Povidone, hydroxyethylcellulose To enhance palatability and mask the taste of the drugs. Eg : Sucrose, saccharin, aspartame Flavouring agents Taste Sensation Recommended flavour Salt Butter scotch, maple, apricot, peach, vanilla, Bitter Wild cherry, walnut, chocolate, mint. Sweet Fruit and berry, vanilla. Sour Citrus flavours, liquorice, raspberry. Colouring agents To enhance the appearance of the vehicle; which matches well with the flavor employed in the preparation .Eg: green with mint, brown with chocolate flavor etc. Manufacturing Consideration Raw Materials : Incoming raw materials should be tested as per specifications that is identity, purity, uniformity and microbial contamination . Equipments : The following types of equipments may be used in the manufacture of liquid formulations:1. Mixing tanks (SS 316 Stainless Steel) equipped with an agitator. 2. Measuring devices for large and small amount of solids and liquids. 3. A filtration system e.g. filter press Cleaning of equipments All equipments must be thoroughly cleaned and sanitized before use. Disinfectants used: Dilute solutions of H2O2, phenol derivatives. Sterilized by: Alcohol, boiling water, autoclaving, steam or dry heat. Manufacturing of Monophasic Liquid Recent Advance in Monophasic Liquid • Recently developed method for the enhancement of solubility of drugs. – – – – Nanocrystal Nanomorph Sonocrystallization Supercritical Fluid Process • Recent advances for the delivery of liquid dosage form – Novel Parenteral drug delivery – Novel ophthalmic drug delivery