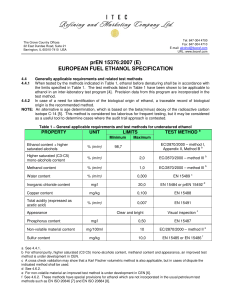
Name : ____________________________________ Strand and Section: __________ Teacher: ________________________________________ Date Submitted: _____________ Graph the heating curve of ethanol using the information given. Use the given graph on the next page. Background Information on Ethanol: Boiling point = 60 oC Melting point = -105 oC Starting temperature = -120 oC 1. After two minutes, frozen cold ethanol starts to melt. It takes two minutes to melt completely. 2. After eight more minutes, it begins to boil. It boils for six minutes. 3. Heat is added for two more minutes until ethanol reaches 80 oC. 4. Label “Melting” where this takes place. 5. Label “Vaporization” where this takes place. 6. Label “Phase Change” where a phase change occurs. 7. Indicate where ethanol is only a SOLID, only a LIQUID, and only a GAS. 8. Of the three phases, label which phase has: Weakest IMF (intermolecular force), Strongest IMF, and Medium IMF.