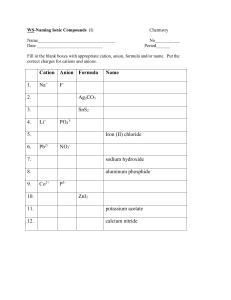
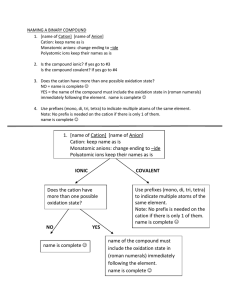
Ionic Bonding/ Formula Units/ Naming Ionic Compounds For each set of elements complete the following data to determine the name and chemical formula of the ionic compound Lewis Dot Structure of each element Cation/ Anion Formation and Oxidation numbers Balance the ionic charges to determine the ratio of atoms in the ionic compound Calculate the Electronegativity Difference between the two types of atoms. Name the compound according to the rules of Naming Sodium and Bromine Magnesium and Chlorine Iron (III) and oxygen Aluminum and Iodine Naming Rules: 1. Name the cation of the compound. ****If the compound is a transition metal provide a Roman numeral to denote the atoms oxidation number. 2. Name the anion and change the ending to “ide” Ionic Bonding Speed Dating: Directions: 1. Each student will be given an index card with an element name. Step one is to record your personal information in the box below for your atom. 2. When time is called, you will start your “date” with the element across from you. Together, you must determine what the chemical formula of the new compound would be, the name of the compound, and the electronegativity difference to determine the bond type. Is the pair truly ionic? Name of Your Element: _________________ Number of Valence Electrons: _________ Cation or Anion? _________________ Oxidation Number: ______________ Pair 1: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: _________________ Pair 2: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 3: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 4: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 5: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 6: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 7: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 8: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 9: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 10: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 11: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 12: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 13: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 14: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________ Pair 15: Cation: ______________ Anion: __________ Chemical formula: ____________ Name: __________________