Ion Formation Worksheet
advertisement
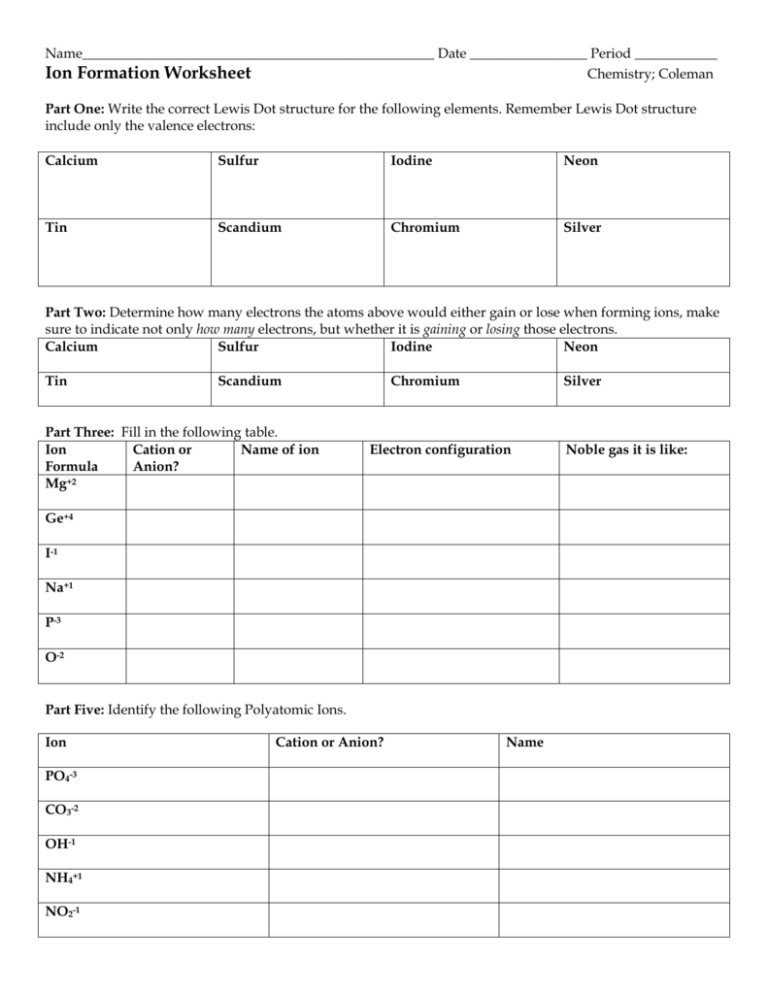
Name___________________________________________________ Date _________________ Period ____________ Ion Formation Worksheet Chemistry; Coleman Part One: Write the correct Lewis Dot structure for the following elements. Remember Lewis Dot structure include only the valence electrons: Calcium Sulfur Iodine Neon Tin Scandium Chromium Silver Part Two: Determine how many electrons the atoms above would either gain or lose when forming ions, make sure to indicate not only how many electrons, but whether it is gaining or losing those electrons. Calcium Sulfur Iodine Neon Tin Scandium Part Three: Fill in the following table. Ion Cation or Name of ion Formula Anion? Mg+2 Chromium Silver Electron configuration Ge+4 I-1 Na+1 P-3 O-2 Part Five: Identify the following Polyatomic Ions. Ion PO4-3 CO3-2 OH-1 NH4+1 NO2-1 Cation or Anion? Name Noble gas it is like: Part Six: Form ionic compounds. Figure out how many of each cation and each anion would need to be combined to form an neutral ionic compound then write the formula for that compound and name it. Remember to include roman numerals for the transition metals. Ions Ex: Na+1 and Se-2 Cu+1 and NO2-1 Mg+2 and I-2 Ga+3 and Te-2 Ge+4 and O-2 Li+1 and OH-1 Number of cations needed 2 Number of anions needed 1 Ionic compound formed Na2Se Name of compound Sodium Selenide











