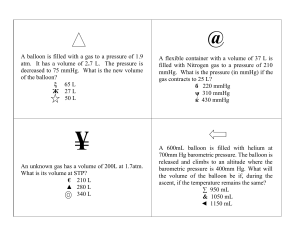
1. A container contains three gases, N2, O2 and Ar, with partial pressures of 23.3 kPa, 40.9 kPa and 13.7 kPa respectively. What is the total pressure inside the container? 2. Under the same conditions of temperature and pressure, how many times faster will hydrogen effuse compared to carbon dioxide? 3. A 25.5 liter balloon holding 3.5 moles of carbon dioxide leaks. If we are able to determine that 1.9 moles of carbon dioxide escaped before the container could be sealed, what is the new volume of the container? 4. What is the partial pressure of water vapor in an air sample when the total pressure is 1.00 atm, the partial pressure of nitrogen is 0.79 atm, the partial pressure of oxygen is 0.20 atm, and the partial pressure of all other gases in the air is 3.34 mmHg? 5. Sally adds 3.13 moles of argon to a 5.29 liter balloon that already contained 2.51 moles of argon. What is the volume of the balloon after the addition of the extra gas? 6. An unknown gas diffuses 4.0 times faster than O2. Find its molar mass.