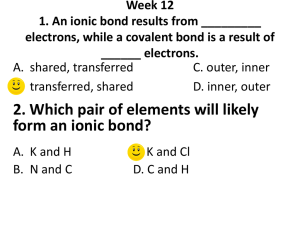
Practicing Ionic Bonds For each compound below: 1. draw each element showing their electrons 2. Use an arrow to show the transfer of electrons from one electron to another a. THINK! How many electrons does your atom need to lose/gain? Do you need more than one type? 3. Write the charge of the ion a. Is it + or -? Whats the size of the charge? 4. Write the chemical formula for the ionic compound a. How many of each atom do you have? b. Write it as: Cation Anion 5. What is the name of the ionic compound Pair 1: Sodium and Chlorine Element Sodium Chlorine Drawing Charge Chemical Formula Name +1 NaCl Sodium Chloride -1 Pair 2: Sodium and Oxygen Element Sodium Oxygen Drawing Charge Chemical Formula Name + Na2O Sodium Oxide -2 Pair 3: Magnesium and Chlorine Element Magnesium Chlorine Drawing Charge Chemical Formula Name 2+ MgCl2 Magnesium Chloride 1- Challenge! Aluminium and Oxygen Element Aluminium Oxygen Drawing Charge Chemical Formula Name 3+ Al2O3 Aluminium Oxide Further practice! (draw in your books) 1. Potassium and Flourine 2. Beryllium and Chlorine 3. Magnesium and Oxygen 2-