Week 12 - Effingham County Schools
advertisement

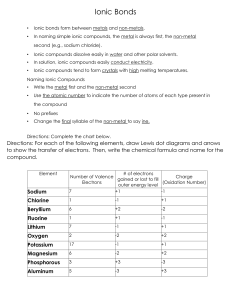
Week 12 1. An ionic bond results from _________ electrons, while a covalent bond is a result of ______ electrons. A. shared, transferred C. outer, inner B. transferred, shared D. inner, outer 2. Which pair of elements will likely form an ionic bond? A. K and H B. N and C C. K and Cl D. C and H 3. How many atoms of oxygen are present in one molecule of H2SO4? A. 1 B. 2 C. 4 D. 8 4. Which pair of elements has the most physical and chemical properties in common? a. Ge and Se b. K and Kr c. Al and Ga d. Ba and Bi 5. Silver is a transition metal that carries a charge of +1 as an ion. Bromine is a halogen that produces an ion with a -1 charge. Which of the following chemical formulas correctly represents 2 molecules of silver bromide? A. 2AgBr C. Ag2Br2 B. Ag(Br)2 D. 2Ag2Br2 6. What is the chemical formula stable ionic compound formed between magnesium and nitrogen? A. MgN B. Mg2N3 C. Mg3N2 D. MgN2 7. The compound formed from the elements calcium and chlorine is known as A. Chlorine calcide B. Calcium dichloride C. Calcium chloride D. Calcium chlorate 8. What is the correct formula for the compound lithium oxide? a. b. c. d. LiO Li2O LiO2 Li2O2 9. When barium metal reacts with chlorine gas it forms an ionic compound. What is the chemical formula for barium chloride? a. b. c. d. BaCl Ba2Cl Ba2Cl2 BaCl2 10. Magnesium loses two electrons to form magnesium ions. Chlorine gains one electron to form chloride ions. Select the correct formula for magnesium chloride. A. B. C. D. MgCl Mg2Cl MgCl2 Mg2Cl2