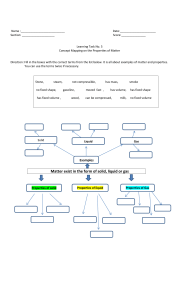
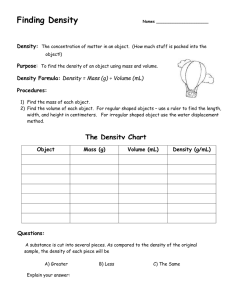
density review worksheet
advertisement

Name ______________________________ Date _________ Block ______ Use the density formula to solve the following problems. Show all work and the answer must have the correct units. Remember that volume can have different forms. (mL or cm³) 1. What is the density of CO gas if 0.196 g occupies a volume of 100 ml? Answer_________ 2. A block of wood 3 cm on each side has a mass of 27 g. What is the density of the block? (Hint, don’t forget to find the volume of the wood.) Answer_________ 3. An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. The height of the water rose to 7 ml. If the mass of the stone was 25 g, what was its density? Answer_________ 4. A 10.0 cm3 sample of copper has a mass of 89.6 g. What is the density of copper? Answer_________ Density is defined as…. ___________________________________________________________________ ___________________________________________________________________ 5. 6. How do you label density? ___________________________________________________________________ ___________________________________________________________________ 7. Anything with a density higher than 1g/mL will ___________________________________________________________________ 8. What are all of the tools needed to find the density of a cube? ___________________________________________________________________ ___________________________________________________________________ 9. What are the tools needed to find the volume of an irregular shaped solid? ___________________________________________________________________ ___________________________________________________________________ 10. True or False? A solid is denser than a liquid. _______________________ 11. True or False? In order to find the density of an object you must have water. ___________________________________________________________________ 12. Density requires the measurements of… ___________________________________________________________________ 13. To prepare for this test I am going to… ___________________________________________________________________ ___________________________________________________________________