1. Suggest explanations for the following observations concerning
advertisement
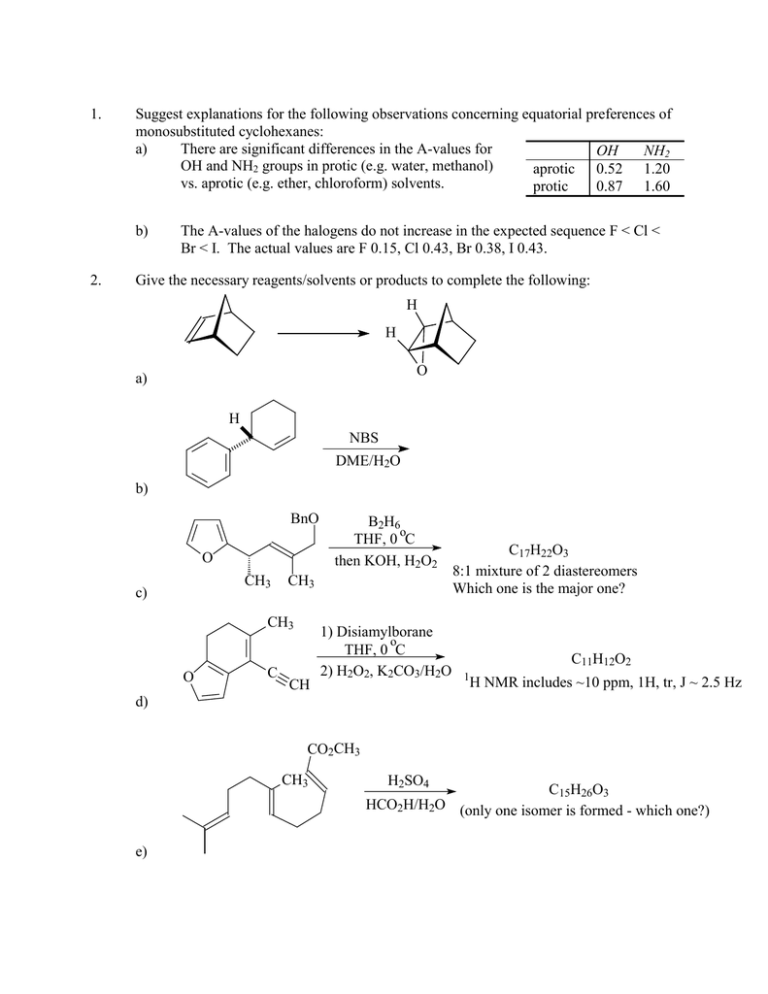
1. Suggest explanations for the following observations concerning equatorial preferences of monosubstituted cyclohexanes: a) There are significant differences in the A-values for OH NH2 OH and NH2 groups in protic (e.g. water, methanol) aprotic 0.52 1.20 vs. aprotic (e.g. ether, chloroform) solvents. protic 0.87 1.60 b) 2. The A-values of the halogens do not increase in the expected sequence F < Cl < Br < I. The actual values are F 0.15, Cl 0.43, Br 0.38, I 0.43. Give the necessary reagents/solvents or products to complete the following: H H O a) H NBS DME/H2O b) BnO O CH3 c) CH3 CH3 O C B2H6 o THF, 0 C then KOH, H2O2 CH 1) Disiamylborane o THF, 0 C 2) H2O2, K2CO3/H2O C17H22O3 8:1 mixture of 2 diastereomers Which one is the major one? C11H12O2 1 H NMR includes ~10 ppm, 1H, tr, J ~ 2.5 Hz d) CO2CH3 CH3 e) H2SO4 C15H26O3 HCO2H/H2O (only one isomer is formed - which one?) 3. Propose a synthetic sequence for the following transformations. More than one step will be needed. O OH a) O NH b) 1. Suggest explanations for the following observations concerning equatorial preferences of monosubstituted cyclohexanes: a) There are significant differences in the A-values for OH NH2 OH and NH2 groups in protic (e.g. water, methanol) aprotic 0.52 1.20 vs. aprotic (e.g. ether, chloroform) solvents. protic 0.87 1.60 b) The A-values of the halogens do not increase in the expected sequence F < Cl < Br < I. The actual values are F 0.15, Cl 0.43, Br 0.38, I 0.43. ANSWER: Part a: The A-value is a measure of the “effective size” of a substituent on a sixmembered ring. It primarily reflects the 1,3-synaxial interactions between the substituent (when it is in an axial orientation) and the axial H atoms. Thus, if the A-value for a group increases on changing solvent, something is apparently making that group behave as if it is physically bigger. Note that both OH and NH2 are hydrogen-bonding groups, and that likewise protic solvents are hydrogen-bonding molecules while aprotic solvents are not. If an OH or NH2 group is hydrogen bonded to one or several solvent molecules, these will form a tight solvation sphere around that group. This sphere will encounter steric problems from the synaxial H atoms if the group is axial. Therefore, it is not surprising that in protic solvents, cyclohexanols and cyclohexylamines have larger equatorial preferences than they do in aprotic solvents. Part b: There is no doubt that the atomic radii of the halogens increase going from F to I. However, their A-values do not reflect this – apparently Cl, Br and I behave more or less as if they were the same size. Recall that as the radius of the atom increases, the bond length also increases. This puts the steric bulk of the atom further away from the 1,3-synaxial hydrogens when it is axially oriented. This effect seems to level out the steric conflicts between the heavier halogens and H-atoms in a cyclohexyl system. 2. Give the necessary reagents/solvents or products to complete the following: H 1) NBS, DMSO/H2O H 2) NaH, THF O a) The point here is that the epoxide is on the more hindered face of the molecule. Thus, peroxyacids will not be suitable. Formation of the bromohydrin proceeds by addition of Br+ on the less hindered face, so that oxygen appears on the more hindered face. Base treatment gives the cyclic ether. H H NBS DME/H2O OH Br b) The preferred conformation of the cyclohexene will probably position the bulky phenyl ring pseudo-axially to avoid allylic A1,2 strain. This means that the bromonium ion will form preferentially on the "top" face of the alkene, anti to the phenyl group. Trans diaxial opening of this bridged ion gives this halohydrin isomer. BnO O CH3 OH BnO B2H6 o THF, 0 C then KOH, H2O2 OH B + O CH3 CH3 O CH3 CH3 C Major product Hydroboration/oxidation occurs by syn addition, with B attached at the less-hindered end of the alkene. The facial selectivity is controlled by the conformation at the allylic position. The preferred conformer will be: BnO CH3 O In this geometry, the allylic C-H bond eclipses the C=C, because there is too much steric hindrance for the furanyl ring or the CH3 group to do so. The furanyl ring blocks approach to the "top face" of the alkene. c) CH3 O C CH 1) Disiamylborane o THF, 0 C 2) H2O2, K2CO3/H2O 1 d) CH3 H CH3 O CH2 CHO H NMR includes ~10 ppm, 1H, tr, J ~ 2.5 Hz Hydroboration/oxidation again. In this case, the internal C=C bond is much more sterically hindered than the terminal alkyne. Reaction occurs at the alkyne, but the intermediate enol is unstable with respect to the aldehyde tautomer. The NMR data indicates the presence of an aldehyde C-H, split by an adjacent CH2 group. CO2CH3 CH3 CO2CH3 CH3 OH H2SO4 HCO2H/H2O H H+ H2O CO2CH3 CO2CH3 CO2CH3 CH3 CH3 CH3 H H H e) 3. This reaction proceeds by a mechanism completely analogous to the acid-catalyzed hydration of an alkene. Protonation of the trisubstituted terminal alkene promotes attack by the electron-rich internal alkene. The resulting cation intermediate is attacked by the third alkene, and finally water adds to the resulting cation. All steps are reversible, and so the most-stable isomer is formed: a trans decalin, in which the ester and OH groups are equatorial. Propose a synthetic sequence for the following transformations. More than one step will be needed. O OH OsO4 NMO acetone/H2O MnO2 CH2Cl2 OH OH a) O NH PCl5 ether (aq. workup) NHOH H2SO4 H2O/THF OH b) Jones' Reagent (CrO3, aq. H2SO4) acetone O NH2OH.HCl NaOH (Aq.) EtOH/H2O






