Homework
advertisement

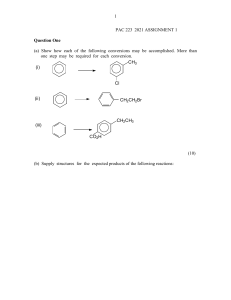
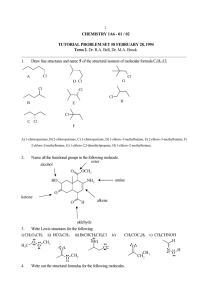
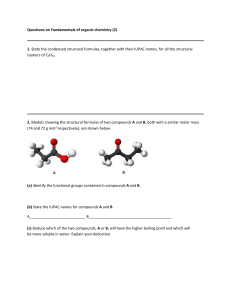
Name: ____________________________ Hour: _____ Date: _____________ AP Chemistry: 25HW Alkanes If given a name, provide the structure. If given a structure or formula, provide the name. 1. 6. bromomethane 2. cyclopropane 7. F F NO2 3. CH3(CH2)2Cl 8. pentane (or n-pentane) 4. 2,3-dimethylbutane 9. 5. 10. 4,5-diethyl-2-methylheptane 11. ethylcyclopentane 16. CH3(CH2)4CH3 12. 17. 3-ethylpentane 13. 4-bromo-1,2-diiodocycloheptane 18. 14. 19. 1,3-difluoropropane 15. 2-amino-3-chlorooctane 20. 2,3,4-trimethylpentane Alkenes/Alkynes If given a name, provide the structure. If given a structure or formula, provide the name. 1. 6. 6-chloro-3-heptyne 2. 2,3-difluorocyclobutene 7. F NO2 3. 8. 7-amino-5-ethyl-1-octene 4. 3-nonene 9. 6-ethyl-3-octene Cl 5. Br 10. 4-ethylcycloheptyne 11. 2-methyl-2-butene 16. 12. 17. 2,4-heptadiene 13. CH2CHCH3 18. Br 14. 19. CH3(CH)2C(CH3)3 15. 4,6-diethyl-3,6-decadiene 20. Structural and Geometric Isomers 1. Draw and name the five structural isomers of hexane. 2. Draw each of the following. trans-2-butene 3. Draw and name the six non-cyclic isomers of C5H10. cis-2,3-dichloro-2-pentene Benzene, Phenol, and Toluene If given a name, provide the structure. If given a structure or formula, provide the name. 1. OH 6. fluorobenzene F NH2 CH3 2. 2,4,6-trinitrotoluene 7. Br Br Br Br 3. 8. 4-ethylphenol Br 4. 4-ethyl-1,2-diiodobenzene 9. 5. 10. 3-ethyl-2-propyltoluene Cl CH3 Alcohols, Phenol, and Phenyl If given a name, provide the structure. If given a structure or formula, provide the name. 1. 6. 3-bromo-4-fluoro-2-nitrophenol Br I OH 2. 5,6-dichloro-2,3,4-triphenyl-2-nonene 7. OH 3. 8. 2-nitro-1,3-heptanediol Cl 4. 3,4-dichloro-2-butanol 9. Br OH OH 5. 10. 5-amino-6-phenyl-2-hexyne O2N NH2 The Carbonyl Group: Ketones, Aldehydes, Esters, Carboxylic Acids If given a name, provide the structure. If given a structure or formula, provide the name. O 1. 6. 3-aminobutanal O 2. 1,1,3-tribromo-1,3,3-trifluoropropanone 7. O OH NH2 O 3. 8. butyl pentanoate I O 4. 3-ethyl-2-methylpentanoic acid 9. Cl F 5. O 10. 5-bromo-3-hexanone O 11. 3,3-dichloropropanoic acid O 16. O 12. 17. ethyl heptanoate I I O 13. trifluoroethanal 18. O 14. O 19. 2-chloro-4-phenylhexanoic acid O 15. 6-chloro-4,5-difluoro-3-heptanone 20. OH Functional Groups and Molecular Masses For each structure given, circle and label any of the following functional groups that are present: alcohol, ether, amine, aldehyde, ketone, carboxylic acid, ester, or amide. Then calculate the molecular mass of each structure. Br 1. Br O 2. O O2N O N OH O 3. O Br O 4. N Cl OH O OH 5. 6. O O OH N O O OH 7. O O OH Br Br Br O Cl Cl 8. OH F N O O O HO Organic Reactions Write a balanced equation for each reaction. For simple substances (like water or hydrogen gas), write formulas in your equation; otherwise, draw structures. Below each formula or structure, unless otherwise specified, write the substance’s name. 1. the complete combustion of 3,4-diphenylheptane 2. the addition of hydrogen chloride to 2-butene 3. the addition of water to 2,5-dimethyl-3-hexene 4. the polymerization of tetrachloroethene (Do not write the name of the product.) 5. the condensation reaction between 1-propanol and ethanol (Do not write the name of the main product. Instead, classify the main product based on the functional group that is present.) 6. the elimination reaction between propanoic acid and 1-aminopropane (Do not write the name of the main product. Instead, classify the main product based on the functional group that is present.) 7. the halogenation of ethane with bromine (Show only the first substitution.) 8. the complete hydrogenation of 1,3-octanediene 9. the reaction between propanoic acid and 1-pentanol