8. Heterogeneous reaction • solid – fluid (liquid, gas) • liquid – gas
advertisement
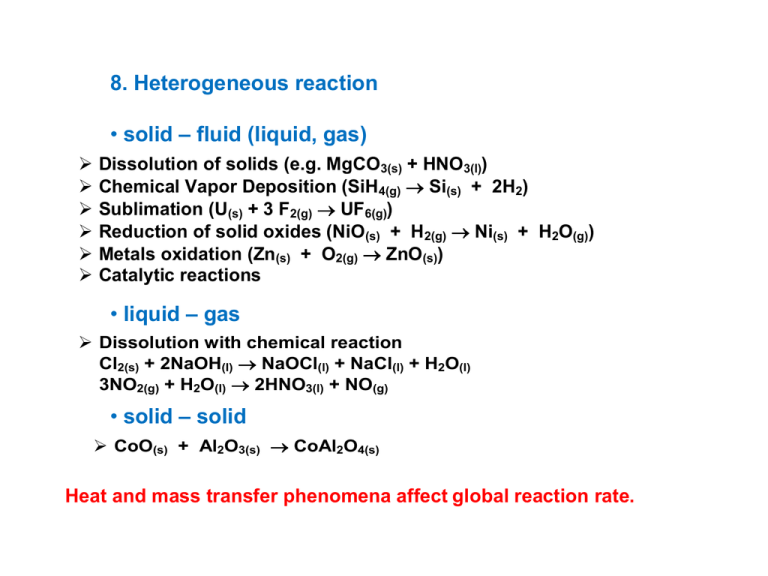
8. Heterogeneous reaction • solid – fluid (liquid, gas) Dissolution of solids (e.g. MgCO3(s) + HNO3(l)) Chemical Vapor Deposition (SiH4(g) Si(s) + 2H2) Sublimation (U(s) + 3 F2(g) UF6(g)) Reduction of solid oxides (NiO(s) + H2(g) Ni(s) + H2O(g)) Metals oxidation (Zn(s) + O2(g) ZnO(s)) Catalytic reactions • liquid – gas Dissolution with chemical reaction Cl2(s) + 2NaOH(l) NaOCl(l) + NaCl(l) + H2O(l) 3NO2(g) + H2O(l) 2HNO3(l) + NO(g) • solid – solid CoO(s) + Al2O3(s) CoAl2O4(s) Heat and mass transfer phenomena affect global reaction rate. Example Si(s) + O2(g) SiO2(s) cO2 t D s O2 2 cO2 porous SiO2 x 2 Si Air (O2) O2 cO2 ( x) co J O( D2 ) flux density of O2 in SiO2 layer (mol.m-2.s-1) c1 Convection + Diffusion k cO2 coefficient of mass transfer of O (m.s-1) 2 rS kc2 N O2 kcO2 [c1 co ] 2 -1 DOs 2 diffusion coefficient of O2 in SiO2 (m .s ) c2 rS x J N O2 flux density of O2 in gas phase(mol.m-2.s-1) diffusion ( D) O2 D s O2 dcO2 dx DOs 2 [c2 c1 ] rate of chemical reaction (mol. m-2.s-1) Steady state NO2 J O( D2 ) O2 rs kcO2 [c1 co ] DOs 2 [c2 c1 ] kc2 DOs 2 kcO2 k c1 co s DO2 k .kcO2 (kcO2 k ) c1 a c2 calculation : DOs 2 DOs 2 c2 kcO2 co kcO2 c1 D k c c D k .k (k k ) DOs 2 DOs 2 c1 k c2 0 SiO2 layer thickness and Si conversion : s O2 cO2 2 cO2 reaction rate rs co 1 (t ) 1 s kcO2 DO2 k M SiO2 , M Si , SiO2 , Si rS SiO d 2 M SiO2 dt o s O2 cO2 1 M SiO2 1 1 2 co t s 2 DO2 SiO2 kcO2 k o M SiO2 Si o X Si o= 2 M Si SiO2 Molar weights and densities of SiO2 a Si o Initial thickness of Si slab 3 limiting cases 1. Rate determining step is the external mass transfer of oxygen towards interface (gas - SiO2) 1 kcO2 , s O2 D 1 kcO2 1 rs kcO2 co , k kcO co 2 M SiO2 SiO cO2 ( x ) t co c1 2 c2 x 2. Rate determining step is the internal mass transfer of oxygen in porous SiO2 layer s O2 D 1 , s O2 kcO2 D DOs 2 1 rs co , k 2D c s O2 o M SiO2 cO2 ( x) t SiO co c1 2 c2 x 3. Rate determining step is chemical reaction taking place on the interface (SiO2 - Si) 1 1 1 s , rs kco , k DO2 k kcO2 Discussion: rS = f(composition), rS = f(temperature) kco M SiO2 SiO cO2 ( x) t co c1 2 c2 x External heat and mass transfer Combustion of the spherical carbon particle Oxygen molar flux at steady state Energy flux at steady state Surface temperature and concentration of oxygen S Two balance equations for unknown c1 , TS Multiple steady state solutions as in the case of CSTR! Example Catalytic reactions • homogeneous catalysis Ozone decomposition in the presence of Cl SO2 oxidation by NOx Esterification catalyzed by acids or bases Enzymatic catalysis O3 + Cl ClO + O2 ClO + O3 Cl + 2O2 2O3 3O2 • heterogeneous catalysis NH3, CH3OH production SO2 to SO3 oxidation HDS, HDN processes Fluid Catalytic Cracking Hydrogenation Polymerization (Ziegler-Natta catalysts, metallocens) Catalytic cycle Non catalytic x catalytic reaction Activated complex of non catalytic reaction Encat – energy of activation for non catalytic reaction Ecat - energy of activation for catalytic reaction Energy Encat ri e Ei RT Ecat Reactants Adsorbed reactants Products Adsorbed products Reaction pathway The multi-step catalytic reaction can be faster than one-step reaction Homogeneous x Heterogeneous Catalysts Homogeneous Heterogeneous All atoms Low No 50-200 oC Limited Surface atoms High (variable) Important 200-1000 oC Large Characterization Structure, composition Modification Temperature stability Well defined Easy Low No clear defintion Difficult High Separation Difficult Easy packed beds Recycling Feasible Feasible Active sites Concentration Diffusion disguises Reaction conditions Application Raw materials (impurities, ..,) Equilibrium Operation conditions (T, P,…) Catalytic reaction Kinetics Properties of Catalyst, Preparation procedure Reactors continuous, batch Hydrodynamics Heat and mass transfer Steps in a catalytic reaction 1) 2) 3) 4) 5) 6) 7) Mass transfer of reactants to the external surface of catalyst Mass transfer of reactants in porous structure of catalyst Adsorption of reactants Surface reaction (+ migration) Desorption of products Mass transfer of products in porous structure of catalyst Mass transfer of reactants from the external surface of catalyst The transport steps (1,2,6,7) depend on T, P, composition, flow rates, pore size, .... The chemical steps (3,4,5) are dependent on T, P, composition. Elementary steps of catalytic reaction Fluid phase Products Reactants Desorption Adsorption Surface reaction Dissociation Migration Mechanisms in heterogeneous catalysis Langmuir-Hinshelwood Rideal-Eley Desorption Adsorption Migration Surface reaction Adsorption x Chemisorption 2 A 2[*] 2 A* A2 2[*] 2A* Adsorption (physical phenomenon) Energy of biatomic molecule A2 Chemisorption (chemical phenomenon) Distance from catalyst surface Emc, Emp – activation energy of migration in adsorbed and chemisorbed state ED – energy of dissociation of molecule A2 Ed – energy of desorption of A2 Ea – energy of activation of transition from adsorbrd to chemisorbed state Adsorption x Chemisorption Adsorption Chemisorption Principal van der Waals forces no electron transfer ! covalent or ionic bonds electron transfer Adsorbent all solids specific sites Adsorbat gases T < Tc reactive components Temperature low higher Enthalpy 10-40 kJ/mol 80-600 kJ/mol Rate high depends on T Activation energy low Occupancy multilayer high monolayer Reversibility YES YES but … Use BET method pore size distribution surface concentration of active sites Chemisorption of fluid phase molecule(adsorbat) Surface occupancy(Θ) Θi = Number of sites occupied by i-th component Total number of sites 0 < Θi < 1 Associative x Dissociative chemisorption Adsorption (chemisorption) isotherm – Surface occupancy as a function of partial pressure of given component at constant temperature Henry isotherm Θi = Hi . pi Irving Langmuir 1920 1932 - adsorption isotherm - kinetics of catalytic reactions on ideal surfaces - Nobel Prize Langmuir adsorption isotherm Brunauer-Emmett-Teller (BET) adsorption isotherm Associative adsorption M(g) + * ka kd M-* * - active site, ka a kd – kinetic constants for adsorption and desorption Adsorption rate = ka . p . (1 - Θ) p = partial pressure of adsorbat Desorption rate = kd . Θ Equilibrium ka . p . (1 - Θ) = kd . Θ Langmuir adsorption isotherm Θ = Ns / N = K . p / (1 + K . p) (K = ka/kd) Asociative chemisorption Langmuir adsorption (chemisorption) isotherm Multicomponent asociative chemisorption A(g) + * ka kd A-* Occupancy of A Occupancy of B KA = ka / kd Irreversible chemisorption B(g) + * kb kd KA.pA ΘA 1 KA.pA KB.pB KB.pB ΘB 1 KA.pA KB.pB KB = kb / kd catalyst poisoning B-* Dissociative chemisorption M2(g) + 2 * ka kd Rate of chemisorption = Rate of desorption k d . Θ2 = ka . p . (1 - Θ)2 = kd . Θ2 Θ2 (1 - Θ)2 = K’ . p 2 M-* ka . p . (1 - Θ)2 (K’ = ka/kd) K'.p Θ 1 K'.p Isotherms Langmuir (chemisorption, adsorption, monolayer, micropores) Θ K.p n* 1 K.p n ads Henry (chemisorption, adsorption, low occupancy) n ads n* Freundlich (chemisorption, adsorption, non ideal) Θ H p n ads n* 1 Θ K pn Θ Aln B p Temkin (chemisorption, non ideal p 1 (C 1) p (p p) nmC nmC p Brunauer-Emmett-Teller (BET) (adsorption, multilayer) nads Virial (adsorption, multilayer ) p (1 a a 2 ...) R.T 0 0 1 2 Brunauer-Emmett-Teller (BET) Extention of Langmuir model Assumptions: adsorption in multilayer s, 1st layer interaction between adsorbent and adsorbat, in 2nd and further layers condensation-like interaction nads p 1 (C 1) p (p p) nmC nmC p 0 0 Mesoporous alumina -1 a (mmol g ) 15 10 5 0 0,0 0,2 0,4 0,6 0,8 1,0 p/po Temperature od calcination : 3,3 nm (450 oC) - 4,5 nm (600 oC) 5,1 nm (800 oC) Reaction rate per mass 1 1 d 1 1 dni rM r m m dt m i dt rM ,k 1 1 dk rk m m dt Reaction rate per surface 1 1 d 1 1 dni rS r S S dt S i dt rS ,k mole.kg 1.s 1 1 1 d k rk S S dt mole.m2 .s 1 Reaction rate per active center (turnover number) 1 1 d 1 1 dni rRS r s 1 nRS nRS dt nRS i dt rRS ,k 1 1 d k rk nRS nRS dt Reaction rate of catalytic reactions Langmuir-Hishelwood ideal surface Rate of elementary steps Rate determining step x steady state hypothesis CO oxidation on Pt o Overall reaction ( Gr 0 ) 2CO(g) + O2(g) 2CO2(g) nebo 2CO(g) + O2(g) 2CO2(g) Pt surface atoms Elementary steps of catalytic CO oxidation on Pt 1. CO chemisorption CO(g) + [*] CO* r1 k f ,1 PCO * kb,1CO 2. O2 dissociative chemisorption O2(g) + 2[*] 2O* * - Pt surface atoms = Catalytic active centre r2 k f ,2 PO2 *2 kb,2O2 3. Surface reaction between CO* and O* CO* + O* *CO2 + * r3 k f ,3CO O kb ,3CO2 * 4. CO2 desorption into gas phase *CO2 CO2(g) + * r4 k f ,4 CO 2 kb ,4 PCO2 * Pi – partial pressures of gaseous components [Pa] i - occupancy (coverage) of the i-th species [-] k f , j , kb , j - reaction rate constants rj – rate of the j-th elementary step [mol/kg katalyzátoru/s mol/molPt/s= 1/s] Rate determining step in steady state ri ri ri Forward reaction rate Fast step Backward reaction rate ri ri step is close to the equilibrium Slow step = Rate determining step Relation between overall reaction rate and the rate of i-th elementary step determines the stoichiometric number i (do not confuse with stoichiometric coefficient !) r ri i i CO(g) + [*] CO* 2 O2(g) + 2[*] 2O* 1 CO* + O* *CO2 + * 2 *CO2 CO2(g) + * 2 2CO(g) + O2(g) 2CO2(g) Rate determining step: surface reaction Reaction rate as a function of measurable variables Example C2H5OH(g) (A1) CH3CHO(g) (A2) + H(g) (A3) catalysts: CuO, CoO a Cr2O3 (Franckaerts J., Froment G.F., Kinetic study of the dehydrogenation of ethanol, Chem. Eng. Sci. 19 (1964) 807-818). Kinetics kK1 P1 P2 P3 / K eq rM 2 1 K1P1 K 2 P2 rM (mol.g-1.hod-1), Pi (bar), k (mol.g-1.hod-1), Keq (bar), Ki (bar-1). Task: to estimate on the basis of experimental data kinetic and adsorption parameters k , K1 , K 2 EXPERIMENTAL SET-UP Franckaerts J., Froment G.F., Kinetic study of the dehydrogenation of ethanol, Chem. Eng. Sci. 19 (1964) 807-818 EXPERIMENTAL DATA W / F1o y1o y4o y2o [g.hod/mol] P [bar] [ ] [ ] [ ] 1,60 0,80 0,40 1,0 1,0 0,40 1,0 0,40 1,60 0,80 0,40 1,0 1,0 0,60 0,80 0,60 1,60 0,80 0,40 1,0 1,0 0,20 0,40 0,40 7,0 4,0 3,0 1,0 1,0 1,0 1,0 10,0 7,0 4,0 3,0 1,0 1,0 1,0 1,0 10,0 7,0 4,0 3,0 1,0 1,0 1,0 10,0 1,0 0,865 0,865 0,865 0,865 0,750 0,865 0,732 0,865 0,865 0,865 0,865 0,865 0,672 0,865 0,672 0,865 0,865 0,865 0,865 0,865 0,672 0,865 0,865 0,865 0,135 0,135 0,135 0,135 0,130 0,135 0,167 0,135 0,135 0,135 0,135 0,135 0,145 0,135 0,145 0,135 0,135 0,135 0,135 0,135 0,145 0,135 0,135 0,135 0,0 0,0 0,0 0,0 0,119 0,0 0,101 0,0 0,0 0,0 0,0 0,0 0,183 0,0 0,183 0,0 0,0 0,0 0,0 0,0 0,183 0,0 0,0 0,0 T X1 o [ C] [ ] 225,0 225,0 225,0 225,0 225,0 225,0 225,0 225,0 250,0 250,0 250,0 250,0 250,0 250,0 250,0 250,0 275,0 275,0 275,0 275,0 275,0 275,0 275,0 275,0 0,066 0,083 0,055 0,118 0,052 0,060 0,052 0,038 0,149 0,157 0,108 0,218 0,123 0,152 0,106 0,094 0,254 0,262 0,20 0,362 0,230 0,118 0,148 0,196 Solution: Minimize the objective function: (k , K1 , K 2 ) 2 NEXP X i 1 exp 1,i X mod 1,i (k , K1 , K 2 ) X1,mod i (k , K1 , K2 ) calculated from isothermal catalytic PFR model: dX1 rM ( X1 ) o d W / F1 W / F1o 0, X 1 0 ATHENA Visual Studio. 225 oC k1 KA1 KA2 250 oC 5.767986E-01 +- 4.112E-01 4.839934E-01 +- 3.591E-01 1.011693E+01 +- 7.376E+00 8.863130E-01 +- 1.668E-01 4.876108E-01 +- 9.443E-02 3.044445E+00 +- 9.054E-01 275 oC 1.675828E+00 +- 4.225E-01 3.803293E-01 +- 1.473E-01 2.812096E+00 +- 1.078E+00 2.5 2 ln(KA2) = f(1/T) y = 7084.1x - 12.075 R² = 0.8181 1.5 1 ln(k1) = f(1/T) 0.5 0 1.80E-03 y = -5802.3x + 11.056 R² = 0.9807 1.85E-03 1.90E-03 1.95E-03 2.00E-03 2.05E-03 -0.5 -1 -1.5 y = 1293.1x - 3.2791 R² = 0.7019 ln(KA1) = f(1/T) Models of catalytic reactors Expanded views of a fixed bed reactor Models of catalytic reactors Model equations involve: • Balance equations for components of reaction mixture in both gas phase and porous catalytical particle • Balance of energy (enthalpy) • Balance of momentum • Flux constitutive equations for component and energy fluxes Pseudo homogeneous Heterogeneous 1-D • withou axial dispersion (pure plug flow) • with axial dispersion Gradients of concentration and temperature between phases 2-D Radial dispersion 1-D pseudo homogeneous model without axial dispersion dp f us K effective diameter of catalytic particle friction coefficient mean fluid velocity overall heat transfer coefficient 1-D pseudo homogeneous model with axial dispersion Boundary conditions Dimensionless form 2-D pseudo homogeneous model with axial and radial dispersion Balance element of volume Balance equations Boundary and initial conditions Fluidized bed catalytic reactor Experimental determination of kinetics of heterogeneous catalytic reactions OVERVIEW 1. Steady state techniques 1.1 Reactors: Batch, CSTR, PFR 1.2 Transport Disguises at Particle Scale 1.3 Kinetic Analysis : Single Reaction, Complex Reactions, Estimation Methods 2. Transient methods 2.1 Dynamical Changes in catalysts: Reaction Sites, Structure and Morphology, Surface Composition 2.2 Modes of Operation: Steps and Pulses, Cycled Feed, Self-sustained Oscillations, TAP, SSITKA 2.3 Modelling and estimation: CO chemisorption on Pt,NO reduction by H2, Electrocatalytic Ethylene Epoxidation, CO methanation 1. Steady state techniques 1.1 Reactors: Batch, CSTR, PFR Screening of catalysts – high throughput methods - most promising formulation investigated in more detail – scale up of the catalytic reaction towards the industrial application starts from the knowledge of detailed reaction kinetics rate equation(s) Batch reactor, CSTR, PFR outlet inlet reactor catalyst silica wadding furnace 700oC Calculation of reaction rate r [mol/kg/s] CSTR PFR + direct evaluation of r.r. BATCH +simple setup +simple setup -outlet condition’s control +variation of m/F -real hydrodynamics differentiation -temperature and conc.gradients - -isothermicity -isothermicity -differentiation in r.r. calculation o A F XA rM A m FAo FA XA FAo rM 1 A dX A d m / FAo 1 n oA dX A rM A m dt 1.2 Transport Disguises at Particle Scale Ideality at reactor scale (plug flow or ideal mixing), it is not sufficient to obtain accurate kinetic data external transfer internal transfer kio L Shi Dmi determ. of mass and heat transfer coefficients determ. of effectiveness factor external transfer Deska Re L 10 5 Re L 105 Shi 0,66 Re 1/ 2 L 1/ 3 i Sc Shi 0,036 Re 0L,8 Sci1 / 3 Koule Shi 2,0 0,4(Re1L/ 2 0,06 Re 2L/ 3 ) Sci0,4 Re L uL Sci Dmi Packed bed k cio M 2 / 3 jD Sci G Sci Dim m G So Re d pG jD f (Re) C. Re n jH Pr h Pr 2 / 3 C pG C pm m m jH f (Re) C Re n ha(Ts To ) ( H r ) rV ( H r )k xAa ( x oA x As ) Ts k xA x oA x As 1 ( H r ) (1 o ) To hTo xA rV c As 1 o cA k cAac oA rV x As 1 o xA k xAax oA ha(Ts To ) ( H r ) rV ( H r )k xAa ( x oA x As ) Ts k xA x oA x As 1 ( H r ) (1 o ) To hTo xA Criteria for the absence of transport limitations (packed bed) c As c oA c o A 0,95 Ts To 0,02 To Experimental tests m / F konst o A XA FAo N i k gi ( cio cis ) ci x T s x J is Di J Hs ( H r ) Di ci ( ro ) ci ci T i T ( ro ) i sT ( ro ) ci ( ro ) ci ( ro ) N H h (To Ts ) cis ci ( ro ) d 2 ci a dci vi rV (T , ck ) Di 2 dx x dx i Prater number Ts T ( ro ) 0 a= 1 2 d 2T a dT ( H r ) rV (T , ck ) s 2 dx x dx x=0 x = ro dc Di i k gi (cio - c si ) dci dT 0 0 dx dx dx dT s h (To Ts ) dx plate cylinder sphere Experimental tests (supposing that external gradients are negligible) m / F konst o A XA 1/ d p 1.3 Kinetic Analysis : Single Reaction, Complex Reactions, Estimation Methods The differential or integral method of kinetic analysis LHHW kinetics XA Linearizationlinear regression Non-linear modelsnonlinear regression o A m/F Example Ethanol dehydrogenation (Franckaerts, Froment, 1964) AR+S C2H5OH(g) CH3CHO (g) + H2 (g) r.d.s. rM Adsorption of A k A ( PA PR PS / K ) KA PR PS K R PR K S PS K w Pw K kK A ( PA PR PS / K ) rM 1 K A PA K R PR K S PS K w Pw 2 1 Surface reaction Desorption of R k R KK R ( PA / PS PR / K ) rM 1 K A PA KK R PA / PS K S PS K w Pw M ( ) X i f i ( ) i 1 2 Example Air oxidation of o-xylene on V2O5 (Vanhove, Froment 1975) o-xylene r2 r1 tolulaldehyde r3 phtalic anhydrid CO, CO2 Estimation methods y f(x, β) e j β y j f(x j , β) j 1, NEXP Moment matrix of residuals M(β) NEXP e j βe j β T j 1 Gradient, GaussNewton Objective functions (β) TraceQM(β (β) Trace V 1M(β (β) detM(β MarquardtLevenberg Quadratic expansion 2. Transient methods Reaction sites - single crystal surfaces, ultra high vacuum structure sensitive reaction rate (Dahl et al. 1999: rate of dissociative adsorption of nitrogen on Ru (0001) surface was reduced by more than nine orders. Structure and Morphology – CO oxidation on the Pt(110) surface. Ertl (1994) has shown by photo-emission electron microscopy that surface concentrations of reacting species vary with time and 2D-space. Grunwaldt (2000) found that structure of Cu particles depend on the reduction potential of gas mixture CO+H2. Surface composition – surface composition of catalyst may change with gas composition – redox systems (Mars, Krevelen, 1953)