Document 13490169
advertisement
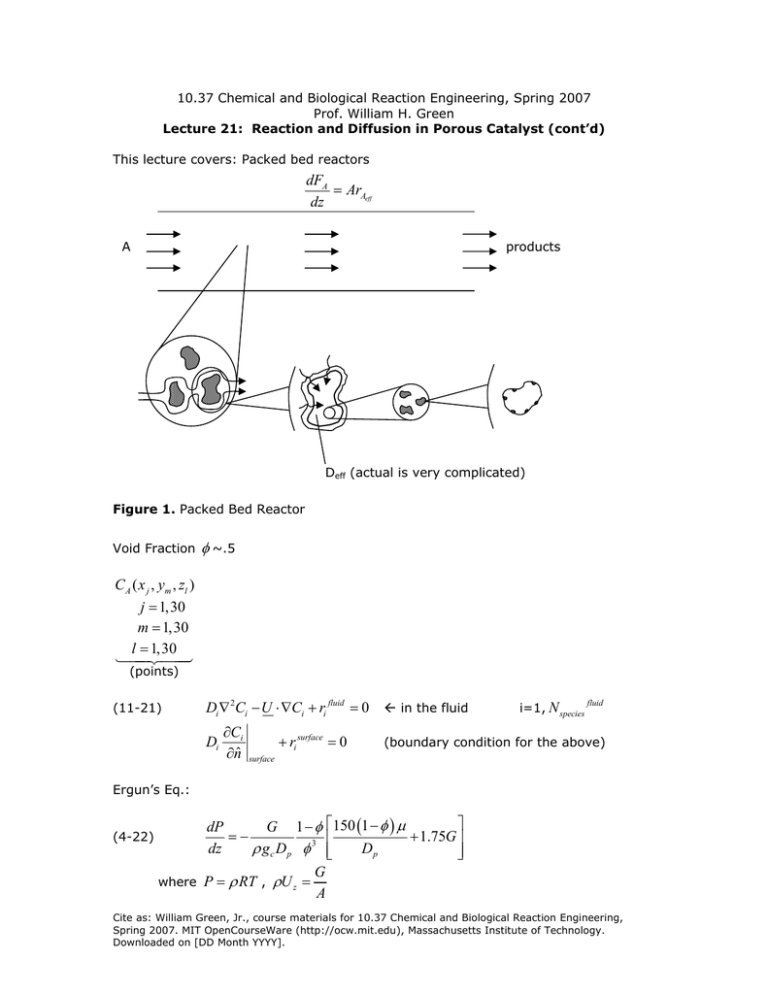
10.37 Chemical and Biological Reaction Engineering, Spring 2007 Prof. William H. Green Lecture 21: Reaction and Diffusion in Porous Catalyst (cont’d) This lecture covers: Packed bed reactors dFA = ArAeff dz A products Deff (actual is very complicated) Figure 1. Packed Bed Reactor Void Fraction φ ~.5 C A ( x j , ym , zl ) j = 1,30 m = 1,30 l = 1,30 (points) (11-21) Di ∇ 2Ci − U ⋅∇Ci + ri fluid = 0 Å in the fluid Di ∂Ci ∂nˆ + ri surface = 0 i=1, N species fluid (boundary condition for the above) surface Ergun’s Eq.: (4-22) dP G 1−φ =− dz ρ gc Dp φ 3 where P = ρ RT , ρU z = ⎡150 (1 − φ ) μ ⎤ + 1.75G ⎥ ⎢ Dp ⎢⎣ ⎥⎦ G A Cite as: William Green, Jr., course materials for 10.37 Chemical and Biological Reaction Engineering, Spring 2007. MIT OpenCourseWare (http://ocw.mit.edu), Massachusetts Institute of Technology. Downloaded on [DD Month YYYY]. ∂FA = ArAeff ∂z rAeff = (effectiveness factor )rAC Ab ( z ) ( ) Ω C Ab ( z ) (or η internal) area total Ω≡ ∂Fi = Ari eff = AΩri ideal ∂z Actual rate of reaction Rate if ri ideal [=] (rAeff ) C A = C Abulk ( z ), and T = Tbulk everywhere mol vol. s ⎛ wt. of catalyst in reactor ⎞ ri ideal = ri′ ⎜ ⎟ V reactor ⎝ ⎠ ri = ri′ ρc (1 − φ ) ⎛ surface area of cat. ⎞ = ri′′ ⎜ ⎟ ρ c (1 − φ ) wt. cat. ⎝ ⎠ ac / m particle m2 [=] Sa + (macroscopic surface area, visual) g FA = ν C A = AUC A (some approximation) Dispersion dC A d 2C A 1 dFA = +U − Da A dz dz dz 2 hope this is 0! Figure 2. Flow over a sphere 10.37 Chemical and Biological Reaction Engineering, Spring 2007 Prof. William H. Green Lecture 21 Page 2 of 3 Cite as: William Green, Jr., course materials for 10.37 Chemical and Biological Reaction Engineering, Spring 2007. MIT OpenCourseWare (http://ocw.mit.edu), Massachusetts Institute of Technology. Downloaded on [DD Month YYYY]. ( ) kc ac C Ab − C As = η ( C As ) rA particle ( C As ) V p Dinside ∂ 2C A + rA ( C A (r ) ) = 0 ∂r 2 ∂C A =0 ∂r r =0 kc C A r = R + Deff inside ∂C A ∂r Deff inside ∂C A = kc C Ab − C As ∂r ( ) = kc C A bulk r=R Matlab: 1) Guess C As ( C A surface) 2) Use boundary conditions to get corresponding 3) Solve ODE (ode15s) 4) Vary guess ( C As ) to make ∂C A ∂r r=R ∂C A = 0 at center ∂r 1st oder irrev. η Ω= 1+ η k1′′ Sa ρb η= 3 φ12 (φ1 coth(φi ) − 1) kc ac φ1 = … k1′′ 10.37 Chemical and Biological Reaction Engineering, Spring 2007 Prof. William H. Green Lecture 21 Page 3 of 3 Cite as: William Green, Jr., course materials for 10.37 Chemical and Biological Reaction Engineering, Spring 2007. MIT OpenCourseWare (http://ocw.mit.edu), Massachusetts Institute of Technology. Downloaded on [DD Month YYYY].