combined approaches for closed-‐loop glucose control
advertisement
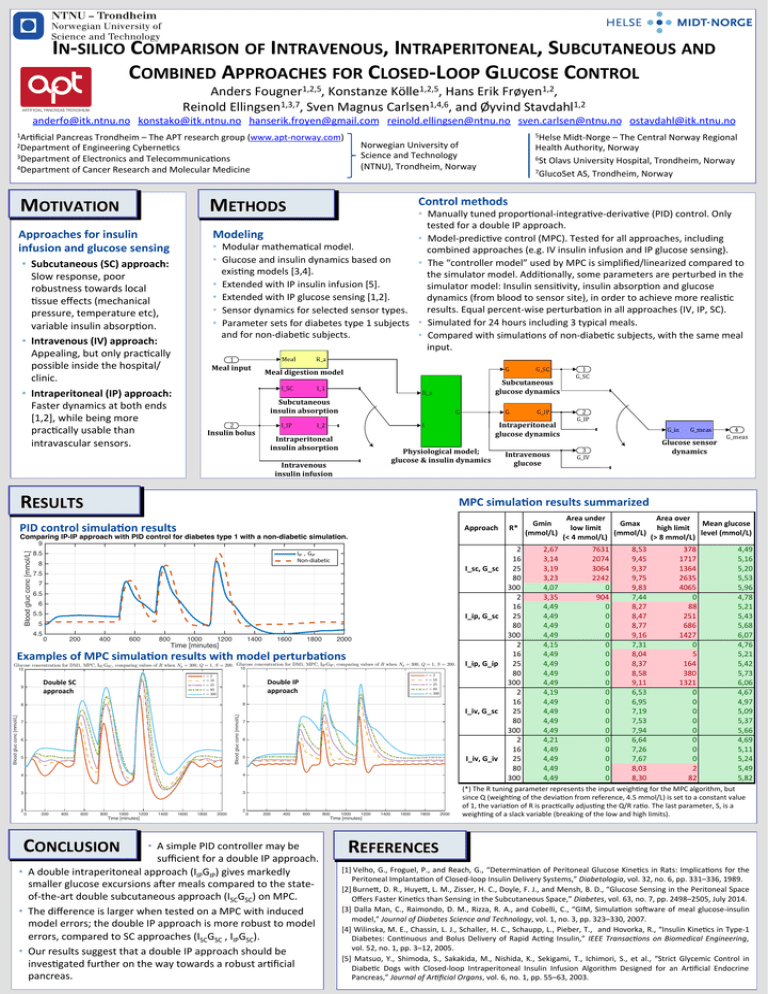
IN-­‐SILICO COMPARISON OF INTRAVENOUS, INTRAPERITONEAL, SUBCUTANEOUS AND COMBINED APPROACHES FOR CLOSED-­‐LOOP GLUCOSE CONTROL Anders Fougner1,2,5, Konstanze Kölle1,2,5, Hans Erik Frøyen1,2, Reinold Ellingsen1,3,7, Sven Magnus Carlsen1,4,6, and Øyvind Stavdahl1,2 anderfo@itk.ntnu.no konstako@itk.ntnu.no hanserik.froyen@gmail.com reinold.ellingsen@ntnu.no sven.carlsen@ntnu.no ostavdahl@itk.ntnu.no 1Ar4ficial Pancreas Trondheim – The APT research group (www.apt-­‐norway.com) Norwegian University of Science and Technology (NTNU), Trondheim, Norway 2Department of Engineering Cyberne4cs 3Department of Electronics and Telecommunica4ons 4Department of Cancer Research and Molecular Medicine MOTIVATION 5Helse Midt-­‐Norge – The Central Norway Regional METHODS Health Authority, Norway 6St Olavs University Hospital, Trondheim, Norway 7GlucoSet AS, Trondheim, Norway Control methods • Manually tuned propor4onal-­‐integra4ve-­‐deriva4ve (PID) control. Only tested for a double IP approach. Modeling • Model-­‐predic4ve control (MPC). Tested for all approaches, including • Modular mathema4cal model. combined approaches (e.g. IV insulin infusion and IP glucose sensing). • Glucose and insulin dynamics based on • The “controller model” used by MPC is simplified/linearized compared to exis4ng models [3,4]. the simulator model. Addi4onally, some parameters are perturbed in the • Extended with IP insulin infusion [5]. simulator model: Insulin sensi4vity, insulin absorp4on and glucose • Extended with IP glucose sensing [1,2]. dynamics (from blood to sensor site), in order to achieve more realis4c results. Equal percent-­‐wise perturba4on in all approaches (IV, IP, SC). • Sensor dynamics for selected sensor types. • Parameter sets for diabetes type 1 subjects • Simulated for 24 hours including 3 typical meals. and for non-­‐diabe4c subjects. • Compared with simula4ons of non-­‐diabe4c subjects, with the same meal input. Approaches for insulin infusion and glucose sensing • Subcutaneous (SC) approach: Slow response, poor robustness towards local 4ssue effects (mechanical pressure, temperature etc), variable insulin absorp4on. • Intravenous (IV) approach: Appealing, but only prac4cally possible inside the hospital/ clinic. Meal 1 Meal input R_a I_SC • Intraperitoneal (IP) approach: Faster dynamics at both ends [1,2], while being more prac4cally usable than intravascular sensors. G Meal digestion model I_1 Subcutaneous glucose dynamics R_a Subcutaneous insulin absorption I_IP 2 Insulin bolus G G Intraperitoneal insulin absorption Physiological model; glucose & insulin dynamics Intravenous insulin infusion RESULTS Intravenous glucose Approach R* Comparing IP-IP approach with PID control for diabetes type 1 with a non-diabetic simulation. Blood gluc conc [mmol/L] 9 IIP , GIP Non-diabetic 8.5 8 I_sc,.G_sc 7.5 7 6.5 6 5.5 I_ip,.G_sc 5 0 200 400 600 800 1000 1200 1400 Time [minutes] 1600 1800 2000 Examples of MPC simula6on results with model perturba6ons Glucose concentration for DM1, MPC, ISC GSC , comparing values of R when Np = 300, Q = 1, S = 200. Glucose concentration for DM1, MPC, IIP GIP , comparing values of R when Np = 300, Q = 1, S = 200. 10 10 r r r r r Double SC approach 9 =2 = 16 = 25 = 80 = 300 I_ip,.G_ip =2 = 16 = 25 = 80 = 300 8 Blood gluc conc [mmol/L] Blood gluc conc [mmol/L] r r r r r Double IP approach 9 8 7 6 5 I_iv,.G_sc 7 6 4 3 3 0 200 400 600 800 1000 1200 1400 1600 1800 2000 I_iv,.G_iv 5 4 2 1 G_SC 2 G_IP G_in G_meas Glucose sensor dynamics 3 G_IV 4 G_meas MPC simula6on results summarized PID control simula6on results 4.5 G_IP Intraperitoneal glucose dynamics I I_2 G_SC 2 0 200 400 600 800 Time [minutes] CONCLUSION • A simple PID controller may be sufficient for a double IP approach. • A double intraperitoneal approach (IIPGIP) gives markedly smaller glucose excursions afer meals compared to the state-­‐ of-­‐the-­‐art double subcutaneous approach (ISCGSC) on MPC. • The difference is larger when tested on a MPC with induced model errors; the double IP approach is more robust to model errors, compared to SC approaches (ISCGSC , IIPGSC). • Our results suggest that a double IP approach should be inves4gated further on the way towards a robust ar4ficial pancreas. 1000 1200 1400 1600 1800 2000 Time [minutes] 2 16 25 80 300 2 16 25 80 300 2 16 25 80 300 2 16 25 80 300 2 16 25 80 300 Gmin. (mmol/L) 2,67 3,14 3,19 3,23 4,07 3,35 4,49 4,49 4,49 4,49 4,15 4,49 4,49 4,49 4,49 4,19 4,49 4,49 4,49 4,49 4,21 4,49 4,49 4,49 4,49 Area.under. Area.over. Gmax. Mean.glucose. low.limit....... high.limit...... (mmol/L) level.(mmol/L) (<.4.mmol/L) (>.8.mmol/L) 7631 2074 3064 2242 0 904 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 0 8,53 9,45 9,37 9,75 9,83 7,44 8,27 8,47 8,77 9,16 7,31 8,04 8,37 8,58 9,11 6,53 6,95 7,19 7,53 7,94 6,64 7,26 7,67 8,03 8,30 378 1717 1364 2635 4065 0 88 251 686 1427 0 5 164 380 1321 0 0 0 0 0 0 0 0 2 82 4,49 5,16 5,20 5,53 5,96 4,78 5,21 5,43 5,68 6,07 4,76 5,21 5,42 5,73 6,06 4,67 4,97 5,09 5,37 5,66 4,69 5,11 5,24 5,49 5,82 (*) The R tuning parameter represents the input weigh4ng for the MPC algorithm, but since Q (weigh4ng of the devia4on from reference, 4.5 mmol/L) is set to a constant value of 1, the varia4on of R is prac4cally adjus4ng the Q/R ra4o. The last parameter, S, is a weigh4ng of a slack variable (breaking of the low and high limits). REFERENCES [1] Velho, G., Froguel, P., and Reach, G., “Determina4on of Peritoneal Glucose Kine4cs in Rats: Implica4ons for the Peritoneal Implanta4on of Closed-­‐loop Insulin Delivery Systems,” Diabetologia, vol. 32, no. 6, pp. 331–336, 1989. [2] Burneo, D. R., Huyeo, L. M., Zisser, H. C., Doyle, F. J., and Mensh, B. D., “Glucose Sensing in the Peritoneal Space Offers Faster Kine4cs than Sensing in the Subcutaneous Space,” Diabetes, vol. 63, no. 7, pp. 2498–2505, July 2014. [3] Dalla Man, C., Raimondo, D. M., Rizza, R. A., and Cobelli, C., “GIM, Simula4on sofware of meal glucose-­‐insulin model,” Journal of Diabetes Science and Technology, vol. 1, no. 3, pp. 323–330, 2007. [4] Wilinska, M. E., Chassin, L. J., Schaller, H. C., Schaupp, L., Pieber, T., and Hovorka, R., “Insulin Kine4cs in Type-­‐1 Diabetes: Con4nuous and Bolus Delivery of Rapid Ac4ng Insulin,” IEEE Transac9ons on Biomedical Engineering, vol. 52, no. 1, pp. 3–12, 2005. [5] Matsuo, Y., Shimoda, S., Sakakida, M., Nishida, K., Sekigami, T., Ichimori, S., et al., “Strict Glycemic Control in Diabe4c Dogs with Closed-­‐loop Intraperitoneal Insulin Infusion Algorithm Designed for an Ar4ficial Endocrine Pancreas,” Journal of Ar9ficial Organs, vol. 6, no. 1, pp. 55–63, 2003.

