Determining the Molar Volume of a Gas
advertisement

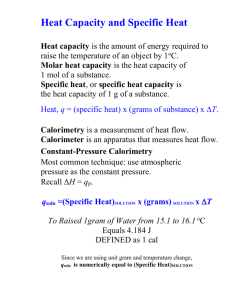
Catalog No. AP6450 Publication No. 6450A Determining the Molar Volume of a Gas AP Chemistry Laboratory #5 Introduction From blimps to airbags, gases are used to fill a wide variety of containers. How much of a particular gas must be produced to fill each of these containers? The amount of gas needed to fill any size container can be calculated if the molar volume of the gas is known. Concepts • Avogadro’s law • Dalton’s law • Ideal gas law • Molar volume Background Avogadro’s law states that equal volumes of gases contain equal numbers of molecules under the same conditions of temperature and pressure. It follows, therefore, that all gas samples containing the same number of molecules will occupy the same volume if the temperature and pressure are kept constant. The volume occupied by one mole of a gas is called the molar volume. In this experiment the molar volume of hydrogen gas at standard temperature and pressure (STP, equal to 273 K and 1 atm) will be measured. The reaction of magnesium metal with hydrochloric acid (Equation 1) provides a convenient means of generating hydrogen in the lab. Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g) Equation 1 If the reaction is carried out with excess hydrochloric acid, the volume of hydrogen gas obtained will depend on the number of moles of magnesium as well as the pressure and temperature. The molar volume of hydrogen can be calculated if the volume occupied by a sample containing a known number of moles of hydrogen is measured. Since the volume will be measured under laboratory conditions of temperature and pressure, the measured volume must be corrected to STP conditions before calculating the molar volume. The relationship among the four gas variables—pressure (P), volume (V), temperature (T), and the number of moles (n)—is expressed in the ideal gas law (Equation 2), where R is a constant called the universal gas constant. PV = nRT Equation 2 The ideal gas law reduces to Equation 3, the combined gas law, if the number of moles of gas is constant. The combined gas law can be used to calculate the volume (V2) of a gas at STP (T2 and P2) from the volume (V1) measured under any other set of laboratory conditions (T1 and P1). In CHEM-FAX姠. . .makes science teaching easier. IN6450A 013107 Determining the Molar Volume of a Gas Page 2 using either the ideal gas law or the combined gas law, remember that temperature must be always be expressed in units of kelvins (K) on the absolute temperature scale. P1V1 P2V2 —— = —— T2 T1 Equation 3 Hydrogen gas will be collected by the displacement of water in an inverted gas measuring tube (also call a eudiometer tube) using the apparatus shown in Figure 1. The total pressure of the gas in the tube will be equal to the barometric (air) pressure. However, the gas in the cylinder will not be pure hydrogen. The gas will also contain water vapor due to the evaporation of the water molecules over which the hydrogen is being collected. According to Dalton’s law, the total pressure of the gas will be equal to the partial pressure of hydrogen plus the partial pressure of water vapor (Equation 4). The vapor pressure of water depends only on the temperature (see Table 1). Ptotal = PH2 + PH2O Equation 4 Eudiometer (Gas Measuring Tube) Hydrogen Gas and Water Vapor Water Magnesium One-hole rubber stopper Figure 1. Table 1. Vapor Pressure of Water at Different Temperatures Temperature, °C PH2O, mm Hg Temperature, °C PH2O, mm Hg 16 °C 13.6 22 °C 19.8 17 °C 14.5 23 °C 21.1 18 °C 15.5 24 °C 22.4 19 °C 16.5 25 °C 23.8 20 °C 17.5 26 °C 25.2 21 °C 18.7 27 °C 26.7 © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Determining the Molar Volume of a Gas Page 3 Experiment Overview The purpose of this experiment is to determine the volume of one mole of hydrogen gas at standard temperature and pressure (STP). Hydrogen will be generated by the reaction of a known mass of magnesium with excess hydrochloric acid in an inverted gas measuring tube filled with water. The volume of hydrogen collected by water displacement will be measured and corrected for differences in temperature and pressure in order to calculate the molar volume of hydrogen at STP. Pre-Lab Questions A reaction of 0.028 g of magnesium with excess hydrochloric acid generated 31.0 mL of hydrogen gas. The gas was collected by water displacement in a 22 °C water bath. The barometric pressure in the lab that day was 746 mm Hg. 1. Use Dalton’s law and the vapor pressure of water at 22 °C (Table 1) to calculate the partial pressure of hydrogen gas in the gas collecting tube. 2. Use the combined gas law to calculate the “corrected” volume of hydrogen at STP. Hint: Watch your units for temperature and pressure! 3. What is the theoretical number of moles of hydrogen that can be produced from 0.028 g of Mg? Hint: Refer to Equation 1 for the balanced equation for the reaction. 4. Divide the corrected volume of hydrogen by the theoretical number of moles of hydrogen to calculate the molar volume (in L/mol) of hydrogen at STP. Materials Copper wire, Cu, 18-gauge, 15-cm long Eudiometer tube, 50-mL Hydrochloric acid, HCl, 2 M, 30 mL Graduated cylinder, 500-mL Magnesium ribbon, Mg, 3.3-cm pieces, 2 Graduated cylinder, 25-mL Distilled or deionized water Metric ruler Wash bottle One-hole rubber stopper, size 1 or 2 Barometer Scissors or wire cutter Beaker, 400-mL Thermometer Safety Precautions Hydrochloric acid is a corrosive liquid. Avoid contact with eyes and skin and clean up all spills immediately. Magnesium metal is a flammable solid. Keep away from flames and other sources of ignition. Wear chemical splash goggles and chemical-resistant gloves and apron. Wash hands thoroughly with soap and water before leaving the laboratory. Procedure 1. Fill a 400-mL beaker about 3⁄4-full with water. 2. Obtain or cut a 3.3-cm piece of magnesium ribbon. Measure and record in the Data Table the exact length of the magnesium ribbon to the nearest 0.1 cm. © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Determining the Molar Volume of a Gas Page 4 3. Your teacher will provide a conversion factor in g/cm to calculate the mass of magnesium used in this experiment. Multiply the length of magnesium ribbon by this conversion factor to calculate the mass of the 3.3-cm piece of magnesium obtained in step 2. Record the mass of magnesium in the Data Table. Copper Wire 4. Obtain a piece of copper wire about 15-cm long. Twist and fold one end of the copper wire around a pencil to make a small “cage” into which the magnesium ribbon may be inserted. See Figure 2. 5. Firmly place the 3.3-cm piece of magnesium into the copper-wire cage. For larger pieces of magnesium, bend or fold the magnesium ribbon first. Magnesium Ribbon 6. Insert the straight end of the copper wire into a one-hole rubber stopper so that the cage end containing the magnesium is about 7-cm below the bottom of the stopper (see Figure 1). Hook the end of the copper wire around the top of the stopper to hold the cage in place. Figure 2. 7. Obtain about 15 mL of 2 M hydrochloric acid in a 25-mL graduated cylinder. 8. While holding the eudiometer tube in a tipped position, slowly add the 2 M hydrochloric acid to the tube. 9. While still holding the eudiometer tube in the tipped position, slowly and carefully fill the tube with distilled or deionized water. Work slowly to avoid mixing the acid and the water layers. Fill the tube all the way to the top so no air remains in the tube. 10. Insert the magnesium–copper wire–stopper assembly into the eudiometer tube. The magnesium piece should be above the 50-mL line on the eudiometer tube (see Figure 3). 11. Place your finger over the hole of the rubber stopper, invert the eudiometer tube, and carefully lower the stoppered end of the tube into the 400-mL beaker containing water. The tube should contain no air bubbles. Clamp the tube in place (see Figure 3). 50-mL mark Figure 3. 12. Record any evidence of a chemical reaction in the Data Table. 13. If the magnesium metal “escapes” its copper cage, gently shake the eudiometer tube up and down to work it back into the acidic solution. Note: Do not lift the tube completely out of the water in the beaker. 14. Allow the apparatus to stand for 5 minutes after the magnesium has completely reacted. Gently tap the sides of the eudiometer tube to dislodge any gas bubbles that may have become attached to the sides. 15. Fill a 500-mL graduated cylinder with tap water and place the cylinder in the sink. © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Determining the Molar Volume of a Gas Page 5 16. Cover the hole in the stopper with your finger and transfer the eudiometer tube to the 500-mL graduated cylinder (see Figure 4). 17. Gently move the eudiometer tube up and down in the cylinder until the water level inside the tube is the same as the water level in the graduated cylinder. This is done to equalize the pressure with the surrounding air (barometric pressure). Note: Make sure the stoppered end of the eudiometer tube remains submerged in the water. 18. When the water levels inside and outside the tube are the same, measure and record in the Data Table the exact volume of hydrogen gas in the eudiometer tube. 19. Measure and record in the Data Table the temperature of the water bath in the graduated cylinder. Using a barometer, measure and record the barometric pressure in the lab in the Data Table. 20. Remove the eudiometer tube from the graduated cylinder and discard the water as directed by your instructor. Figure 4. 21. Repeat the entire procedure to obtain a second set of data. Record this as Trial 2 in the Data Table. If time permits, perform a third trial as well. © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Determining the Molar Volume of a Gas Page 6 Name __________________________________ Data Tables Trial 1 Trial 2 Length of Mg Ribbon Mass of Mg Evidence of Chemical Reaction Volume of H2 Gas Corrected Volume of H2 Temperature of Water Bath (Grad. Cylinder) Barometric Pressure Post-Lab Calculations and Analysis Construct a Results Table to summarize the results of the following calculations. 1. Calculate the theoretical number of moles of hydrogen gas produced in Trials 1 and 2. 2. Use Table 1 in the Background section to find the vapor pressure of water at the temperature of the water bath in this experiment. Calculate the partial pressure of hydrogen gas produced in Trials 1 and 2. 3. Use the combined gas law to convert the measured volume of hydrogen to the “ideal” volume the hydrogen gas would occupy at STP for Trials 1 and 2. Hint: Remember the units! 4. Divide the volume of hydrogen gas at STP by the theoretical number of moles of hydrogen to calculate the molar volume of hydrogen for Trials 1 and 2. © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Determining the Molar Volume of a Gas Page 7 5. What is the average value of the molar volume of hydrogen? Look up the literature value of the molar volume of a gas in your textbook and calculate the percent error in your experimental determination of the molar volume of hydrogen. | Experimental value – Literature value | Percent error = ————————————————— × 100% Literature value 6. One mole of hydrogen gas has a mass of 2.02 g. Use your value of the molar volume of hydrogen to calculate the mass of one liter of hydrogen gas at STP. This is the density of hydrogen in g/L. How does this experimental value of the density compare with the literature value? (Consult a chemistry handbook for the density of hydrogen.) 7. In setting up this experiment, a student noticed that a bubble of air leaked into the graduated cylinder when it was inverted in the water bath. What effect would this have on the measured volume of hydrogen gas? Would the calculated molar volume of hydrogen be too high or too low as a result of this error? Explain. 8. A student noticed that the magnesium ribbon appeared to be oxidized—the metal surface was black and dull rather than silver and shiny. What effect would this error have on the measured volume of hydrogen gas? Would the calculated molar volume of hydrogen be too high or too low as a result of this error? Explain. © 2007 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Determining the Molar Volume of a Gas, Catalog No. AP6450, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Catalog No. AP6450 Publication No. 11195 Determining the Molar Volume of a Gas Inquiry Guidance and AP* Chemistry Curriculum Alignment Introduction Avogadro, Boyle, Charles and Dalton—these scientists and their gas laws are well known. Together their work defined the relationships among four macroscopic gas properties: pressure, volume, temperature and the number of moles of gas. The gas laws have applications in physiology, meteorology, scuba-diving, and even hot-air ballooning or airbag construction. How much gas must be generated to fill a hot air balloon or an airbag? The amount of gas needed to fill any container can be calculated if we know the molar volume of the gas. Answering this general question requires knowledge of all of the gas laws (ABCs +D)! Opportunities for Inquiry Determining the molar volume of a gas is a classic experiment that ties together principles and concepts from several “big ideas” in chemistry. It also reinforces and integrates understanding of key learning objectives, from kinetic molecular theory to the ideal gas law and stoichiometry. Finally, the experiment draws upon and helps students develop science practice skills involving mathematical reasoning and data analysis. Opportunities for inquiry are abundant! The traditional experiment can be transitioned to guided inquiry using some or all of the following approaches, which will improve student preparation and increase the level of student engagement and their ownership of the results. • Take away the data tables and post-lab questions! Replace worksheet calculations with a detailed overview of the experiment describing the general calculations: “The purpose of this experiment is to determine the volume of one mole of hydrogen gas at standard temperature and pressure (STP). Hydrogen will be generated by the reaction of a known mass of magnesium with excess hydrochloric acid in a gas measuring tube filled with water. The volume of hydrogen will be measured by water displacement after adjusting to atmospheric pressure, and must be corrected using (a) Avogadro’s law for the number of moles of gas; (b) Dalton’s law to account for the presence of water vapor in the collected gas; (c) the combined gas law to convert pressure and temperature to STP.” • This is a challenging lab, so make it a challenge! Rather than having students use a prescribed amount of magnesium to generate an unknown volume of gas, challenge them to produce a specific volume of hydrogen gas. Students must work backwards using the gas law calculations described above to calculate how much magnesium metal to use. • Introduce the lab by demonstrating the general setup for generating and collecting a gas. Guide students to design the actual experimental procedure through a series of leading questions. What information (data) is needed to calculate the molar volume of a gas? What variables will influence the experimental data? Choose the independent and dependent variables for the experiment and describe the variables that should be kept constant during the experiment. Students could also discuss variables or other factors that will affect the accuracy of the results and how these may be controlled. • Given the general macroscale procedure, students could design microscale experiments at different temperatures. Compare class results to identify trends in accuracy or deviations from ideal gas behavior as a function of temperature. • Extend the lab to incorporate consumer products, such as analyzing butane gas collected from a lighter. Alignment with AP Chemistry Curriculum Framework—Big Ideas 2 and 3 Enduring Understanding and Essential Knowledge Matter can be described by its physical properties. The physical properties of a substance generally depend on the spacing between the particles (atoms, molecules, ions) that make up the substance and the forces of attraction among them. (Enduring Understanding 2A) 2A2: The gaseous state can be effectively modeled with a mathematical equation relating various macroscopic properties. A gas has neither a definite volume nor a definite shape; because the effects of attractive forces are minimal, we usually assume that the particles move independently. *AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, this product. CHEM-FAX. . .makes science teaching easier. IN11195 040313 he strong electrostatic forces of attraction holding atoms together in a unit are called chemical bonds. (Enduring T Understanding 2C) 2C1: In covalent bonding, electrons are shared between the nuclei of two atoms to form a molecule or polyatomic ion. Electronegativity differences between the two atoms account for the distribution of the shared electrons and the polarity of the bond. hemical changes are represented by a balanced chemical equation that identifies the ratios with which reactants react and C products form. (Enduring Understanding 3A) 3A2: Quantitative information can be derived from stoichiometric calculations that utilize the mole ratios from the balanced chemical equations. The role of stoichiometry in real-world applications is important to note, so that it does not seem to be simply an exercise done only by chemists. hemical and physical transformations may be observed in several ways and typically involve a change in energy. (Enduring C Understanding 3C) 3C1: Production of heat or light, formation of a gas, and formation of a precipitate and/or a color change are possible evidences that a chemical change has occurred. Learning Objectives 2.4 The student is able to use KMT and concepts of intermolecular forces to make predictions about the macroscopic properties of gases, including both ideal and nonideal behaviors. 2.5 The student is able to refine multiple representations of a sample of matter in the gas phase to accurately represent the effect of changes in macroscopic properties on the sample. 2.6 The student can apply mathematical relationships or estimation to determine macroscopic variables for ideal gases. 2.12 The student can qualitatively analyze data regarding real gases to identify deviations from ideal behavior and relate these to molecular interactions. 2.17 The student can predict the type of bonding present between two atoms in a binary compound based on position in the periodic table and the electronegativity of the elements. 3.3 The student is able to use stoichiometric calculations to predict the results of performing a reaction in the laboratory and/or to analyze deviations from the expected results. 3.4 The student is able to relate quantities (measured mass of substances, volumes of solutions, or volumes and pressures of gases) to identify stoichiometric relationships for a reaction, including situations involving limiting reactants and situations in which the reaction has not gone to completions. 3.10 The student is able to evaluate the classification of a process as a physical change, chemical change, or ambiguous change based on both macroscopic observations and the distinction between rearrangement of covalent interactions and noncovalent interactions. Science Practices 1.3 The student can refine representations and models of natural or man-made phenomena and systems in the domain. 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively. 2.2 The student can apply mathematical routines to quantities that describe natural phenomena. 2.3 The student can estimate numerically quantities that describe natural phenomena. 5.1 The student can analyze data to identify patterns or relationships. 6.1 The student can justify claims with evidence. 6.4 The student can make claims and predictions about natural phenomena based on scientific theories and models. 6.5 The student can evaluate alternative scientific explanations. 7.2 T he student can connect concepts in and across domain(s) to generalize or extrapolate in and/or across enduring understandings and/or big ideas. Catalog No. AP6450 Description Determining the Molar Volume of a Gas—AP Chemistry Laboratory 5 Consult your Flinn Scientific Catalog/Reference Manual for current prices. –2– © 2013 Flinn Scientific, Inc. All Rights Reserved. IN11195 Catalog No. AP7654 Publication No. 7654 Designing a Hand Warmer AP* Chemistry Big Idea 5, Investigation 12 An Advanced Inquiry Lab Introduction Put your chemistry skills to commercial use! From instant cold packs to flameless ration heaters and hand warmers, the energy changes accompanying physical and chemical transformations have many consumer applications. The backbone of these applications is calorimetry—measuring heat transfer. Investigate the energy changes accompanying the formation of solutions for common laboratory salts, and then apply the results to design a hand warmer that is reliable, safe, and inexpensive. Concepts • Enthalpy change • Heat of solution • Exothermic versus endothermic • Calorimetry • Specific heat • System and surroundings Background Hand warmers are familiar cold weather gear used to quickly provide warmth to frigid fingers. Many commercial hand warmers consist of a plastic package containing a solid and an inner pouch filled with water. When the pack is activated, the solid dissolves in water and produces a large temperature change. The energy or enthalpy change associated with the process of a solute dissolving in a solvent is called the heat of solution (ΔHsoln). At constant pressure, this enthalpy change, ΔHsoln, is equal in magnitude to the heat loss or gain, q, to the surroundings. In the case of an ionic solid dissolving in water, the overall energy change is the net result of three processes—the energy required to break the attractive forces between ions in the crystal lattice (ΔH1 = + C kJ/mole), the energy required to disrupt intermolecular forces between water molecules (ΔH2 = + D kJ/mole), and the energy released when the dissociated (free) ions form ion-dipole attractive forces with the water molecules (ΔH3 = − F kJ/mole). The overall process can be represented by the following equation. MaXb(s) → aM+b(aq) + bX–a(aq) ΔHsoln = ΔH1 + ΔH2 + ΔH3 = (+ C + D − F) kJ/mole If the amount of energy released in the formation of hydrated ions (ΔH3) is greater than the amount of energy required to separate the solute and solvent particles (ΔH1 + ΔH2), then the sum (ΔHsoln) of the energy changes will be negative and the solution process exothermic (releases heat). If the amount of energy released in the formation of hydrated ions is less than the amount of energy required to separate the solute and solvent particles, then the sum of the energy changes will be positive and the solution process endothermic (absorbs heat). Heats of solution and other enthalpy changes are generally measured in an insulated vessel called a calorimeter that reduces or prevents heat loss to the atmosphere outside the reaction vessel. The process of a solute dissolving in water may either release heat into the resulting aqueous solution or absorb heat from the solution, but the amount of heat exchanged between the calorimeter and the outside surroundings should be minimal. When using a calorimeter, the reagents being studied are mixed directly in the calorimeter and the temperature is recorded both before and after the reaction has occurred. The amount of heat transfer (q) may be calculated using the heat energy equation: q = m × s × ΔT Equation 1 where m is the total mass of the solution (solute plus solvent), s is the specific heat of the solution, and ΔT is the observed temperature change. The specific heat of the solution is generally assumed to be the same as that of water, namely, 4.18 J/gz°C. *AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, this product. CHEM-FAX. . .makes science teaching easier. IN7654 040313 When measuring the heat transfer for an exothermic heat of solution using a calorimeter, most of the heat released is absorbed by the aqueous solution (qaq). A small amount of the heat will be absorbed by the calorimeter itself (qcal). The overall heat transfer (qsoln) for the reaction (the system) then becomes qsoln = –(qaq + qcal) Equation 2 In order to determine the correction factor qcal for heat of solution calculations, the heat capacity of the calorimeter, also called the calorimeter constant, must be determined experimentally. The calorimeter constant has units J/oC. This calibration experiment is done by mixing equal volumes of hot and cool water in the calorimeter and measuring the temperature after 20 seconds. The resulting value is assumed to be the instantaneous mixing temperature, Tmix. The average temperature Tavg of the initial hot (TH) and cool water (TC) is also calculated: Tavg = (TH + TC)/2 The difference between Tavg and Tmix is due to the heat lost by the water and absorbed by the calorimeter. The heat lost by the water, qwater, is: qwater = (mass of water) × (specific heat of water) × (Tmix – Tavg) Equation 3 where the mass is the total mass of hot and cool water. The heat gained by the calorimeter, qcalor, is equal to that lost by the water, but opposite in sign. The calorimeter constant, Ccal, is calculated as follows: qcalor Ccal = ——————– (Tmix – Tinitial) Equation 4 where Tinitial is the initial temperature of the calorimeter containing cool water. To calculate the correction factor qcal for use in Equation 2 above—to determine the heat of solution or heat of reaction for any system—the calorimeter constant is multiplied by the change in temperature of that solution. qcal = ΔT (°C) × Ccal (J/°C) Experiment Overview The purpose of this advanced inquiry lab is to design an effective hand warmer that is inexpensive, nontoxic, and safe for the environment. The investigation begins with an introductory activity to become familiar with the principles of calorimetry and heat of solution calculations. The results provide a model for the guided-inquiry challenge, which is to design an optimum hand warmer for consumer applications. Working in groups of four, each student group will be provided six different solids, along with their costs and individual Material Safety Data Sheets (MSDS). Determine the heat of solution for each solid and analyze the cost and safety information to propose a design for the best all-around hand warmer. Pre-Lab Questions 1. When chromium chloride, CrCl2, is dissolved in water, the temperature of the water decreases. (a) Is the heat of solution exothermic or endothermic? (b) Which is stronger—the attractive forces between water molecules and chromium and chloride ions, or the combined ionic bond strength of CrCl2 and intermolecular forces between water molecules? Explain. 2. A solution was formed by combining 25.0 g of solid A with 60.0 mL of distilled water, with the water initially at 21.4 °C. The final temperature of the solution was 25.3 °C. Calculate the heat released as the solid dissolved, qsoln, assuming no heat loss to the calorimeter (see Equation 1). 3. In Question 2 above, the calorimeter was found to have a heat capacity of 8.20 J/°C. If a correction is included to account for the heat absorbed by the calorimeter, what is the heat of solution, qsoln? 4. The solid in Question 2 was aluminum sulfate, Al2(SO4)3. Calculate the molar heat of solution, ΔHsoln, for aluminum sulfate. Hint: The units for molar heat of solution are kilojoules per mole (kJ/mole). First determine the heat released per gram of solid. –2– IN7654 © 2013 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Designing a Hand Warmer, Catalog No. AP7654, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Materials Ionic solids: Set A: Ammonium nitrate, NH4NO3, 15 g Calorimeter (two nested polystyrene cups) Calcium chloride, anhydrous, CaCl2, 15 g Graduated cylinder, 100-mL Sodium acetate, NaCH3CO2, 15 g Heat-resistant gloves Set B: Sodium chloride, NaCl, 15 g Hot plate Lithium chloride, LiCl, 15 g Magnetic stirrer and stir bar, or stirring rod Sodium carbonate, Na2CO3, 15 g Paper towels Magnesium sulfate, anhydrous, MgSO4, 5 g Support stand and ring clamp Water, deionized or distilled Thermometer, digital Beaker, 250-mL Timer or stopwatch Safety Precautions Ammonium nitrate is a strong oxidizer and may explode if heated under confinement. It is also slightly toxic by ingestion and a body tissue irritant. Calcium chloride is slightly toxic. Lithium chloride is moderately toxic by ingestion. Magnesium sulfate is a body tissue irritant. Sodium acetate is a body tissue and respiratory tract irritant. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Wash hands thoroughly with soap and water before leaving the laboratory. Introductory Activity Part A. Heat Capacity of the Calorimeter Nested polystyrene cups 1. Working in pairs, set up a calorimeter consisting of two nested polystyrene cups in a ring clamp attached to a support stand. Ring clamp 2. Place a magnetic stirrer below the calorimeter, then lower the ring clamp until the bottom of the cup just sits on the surface Stirrer of the magnetic stirrer (see Figure 1). 3. Measure 100.0 mL of distilled water in a 100-mL graduated cylinder and transfer the water into the calorimeter. Stir bar Ring stand Figure 1. 4. Add a magnetic stirring bar to the calorimeter, and set the bar spinning slowly. If a magnetic stirrer is not available, use a stirring rod. Do not remove the stirring rod from the calorimeter. 5. Measure and record the initial temperature of the water. 6. Heat approximately 125 mL of distilled water to about 70 °C in a 250-mL beaker. 7. Using heat-resistant gloves, measure 100.0 mL of the 70 °C distilled water in a 100-mL graduated cylinder. 8. Measure and record the temperature of the hot water. 9. Immediately pour the hot water into the room temperature water in the calorimeter. 10. Insert the thermometer, and stir the water. 11. Record the mixing temperature Tmix after 20 seconds. 12. Empty the calorimeter and dry the inside. 13. Calculate the calorimeter constant, Ccal, using Tmix and Equations 3 and 4 from the Background section. –3– IN7654 © 2013 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Designing a Hand Warmer, Catalog No. AP7654, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Part B. Calorimetry Procedure Working in pairs, examine the heat energy change for the following solution. MgSO4(s) + H2O(l) → Mg2+(aq) + SO42–(aq) 1. Measure 45.0 mL of distilled or deionized water in a 100-mL graduated cylinder and transfer to the calorimeter. 2. Measure and record the initial temperature of the water. 3. Measure 5.00 g of anhydrous magnesium sulfate in a weighing dish. 4. Put a magnetic stir bar or stirring rod into the calorimeter and slowly stir the water. 5. Quickly add the 5.00 g of anhydrous magnesium sulfate to the calorimeter and insert the thermometer. 6. Monitor the temperature and record the highest or lowest temperature reading. 7. Calculate the molar heat of solution for magnesium sulfate. Include the correction due to the heat capacity of the calorimeter. Guided-Inquiry Design and Procedure 1. Review the calorimetry procedure and answer the following questions: a. What data is needed to calculate the enthalpy change for a reaction? b. Identify the variables that will influence the experimental data. c. What variables should be controlled (kept constant) during the procedure? d.The independent variable in an experiment is the variable that is changed by the experimenter, while the dependent variable responds to or depends on the changes in the independent variable. Name the independent and dependent variables in a calorimetry experiment to determine the molar heat of solution. e. Discuss the factors that will affect the precision of the experimental results. 2. Form a working group with four students per group. One pair of students in the group should study the three solids in Set A, while the other pair studies Set B. 3. Working collaboratively with the general procedure provided in the Introductory Activity, design and carry out experiments to determine the heat of solution for each solid. Be sure to review all safety precautions with your instructor before starting. 4. Extrapolating from the information collected, predict which solid(s) could be used in an effective hand warmer meeting the following requirements: • The hand warmer must contain 10 g of an ionic solid and an inner pouch filled with 40 mL of water. • Activating the hand warmer must increase the temperature of the resulting solution by at least 20 °C. 5. Review the cost information shown below and consult the MSDS for each potential hand warmer. Propose the optimum design for the most cost-effective hand warmer that is nontoxic and least harmful to the environment. Solid Cost ($)/Kilogram NH4NO3 CaCl2 LiCl NaCH3CO2 Na2CO3 NaCl 18.50 10.80 68.30 27.30 5.95 4.25 6. With your instructor’s permission, verify the design and demonstrate the use of your hand warmer. Opportunities for Inquiry Instant cold packs are used to treat sports and other injuries when ice is unavailable. Research the properties of commercial cold packs and select a “cold pack solid” from among the solids provided in this activity. Propose and test a design (quantity of solid and volume of water) for an effective instant cold pack. –4– IN7654 © 2013 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Designing a Hand Warmer, Catalog No. AP7654, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. AP Chemistry Practice Review Questions Integrating Content, Inquiry and Reasoning Review the following data from a calorimetry experiment to determine the heat of fusion of ice. After shaking off any excess water, several ice cubes were added to 99 g of warm water contained in a calorimeter. The initial temperature of the warm water was 46.8 °C. The ice−water mixture was stirred until the temperature reached a stable, minimum value, which was 1.1 °C. Any unmelted ice remaining at this point was immediately and carefully removed using tongs and the mass of the water in the calorimeter was measured—154 g. 1. Use the heat energy equation to calculate the amount of heat in joules released by the warm water as it cooled. 2. Based on the law of conservation of energy, what amount of heat was absorbed by the ice as it melted? 3. Determine the amount of energy absorbed per gram of ice as it melted. 4. Calculate the heat of fusion (the heat required to melt ice) in units of kilojoules/mole. 5. The literature value for the heat of fusion of ice is 6.02 kJ/mole. What is the percent error for the experimentally determined heat of fusion? 6. When a mixture of ice and water originally at 0 °C is heated, the temperature remains constant (within experimental error) until all of the ice melts. Explain what happens to the heat energy that is absorbed during this time while the temperature does not change. –5– IN7654 © 2013 Flinn Scientific, Inc. All Rights Reserved. Reproduction permission is granted only to science teachers who have purchased Designing a Hand Warmer, Catalog No. AP7654, from Flinn Scientific, Inc. No part of this material may be reproduced or transmitted in any form or by any means, electronic or mechanical, including, but not limited to photocopy, recording, or any information storage and retrieval system, without permission in writing from Flinn Scientific, Inc. Teacher’s Notes Designing a Hand Warmer Materials Included in Kit (for a class of 24 students working in pairs) Ammonium nitrate, NH4NO3, 120 g Sodium acetate, NaCH3CO2, 120 g Calcium chloride, anhydrous, CaCl2, 120 g Sodium carbonate, Na2CO3, 120 g Lithium chloride, LiCl, 120 g Sodium chloride, NaCl, 120 g Magnesium sulfate, anhydrous, MgSO4, 120 g Cups, polystyrene, 8 oz., 24 Additional Materials Needed (for each lab group) Water, deionized or distilled Magnetic stirrer and stir bar, or stirring rod Balance, 0.01-g precision (shared) Paper towels Beaker, 250-mL Ring clamp and support stand Graduated cylinder, 100-mL Thermometer, digital Hot plate (shared) Timer or stopwatch Safety Precautions Ammonium nitrate is a strong oxidizer and may explode if heated under confinement. It is also slightly toxic by ingestion and a body tissue irritant. Avoid contact of oxidizers with combustible organic materials. Calcium chloride is slightly toxic. Lithium chloride is moderately toxic by ingestion. Magnesium sulfate is a body tissue irritant. Sodium acetate is a body tissue and respiratory tract irritant. Avoid contact of all chemicals with eyes and skin. Wear chemical splash goggles, chemical-resistant gloves, and a chemical-resistant apron. Remind students to wash their hands thoroughly with soap and water before leaving the laboratory. Please review current Material Safety Data Sheets for additional safety, handling, and disposal information. Disposal Please consult your current Flinn Scientific Catalog/Reference Manual for general guidelines and specific procedures, and review all federal, state and local regulations that may apply, before proceeding. The solid samples may be stored for future use or disposed of according to Flinn Suggested Disposal Method #26a.The experimental solutions may be rinsed down the drain with plenty of excess water according to Flinn Suggested Disposal Method #26b. Alignment to AP* Chemistry Curriculum Framework Enduring Understanding and Essential Knowledge hemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molC ecules and the forces between them. 2A3: Solutions are homogenous mixtures in which the physical properties are dependent on the concentration of the solute and the strengths of all interactions among the particles of the solutes. Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons. 3C2: Net changes in energy for a chemical reaction can be endothermic or exothermic. The laws of thermodynamics describe the essential role of energy and explain and predict the direction of changes in matter. 5B3: Chemical systems undergo three main processes that change their energy: heating/cooling, phase transitions, and chemical reactions. 5B4: Calorimetry is an experimental technique that is used to measure the change in energy of a chemical system. –6– © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued Learning Objectives 2.8 The student can draw and/or interpret representations of solutions that show the interactions between the solute and solvent. 3.11 The student is able to interpret observations regarding macroscopic energy changes associated with a reaction or process to generate a relevant symbolic and/or graphical representation of the energy changes. 5.6 The student is able to use calculations or estimations to relate energy changes associated with heating/cooling a substance to the heat capacity, relate energy changes associated with a phase transition to the enthalpy of fusion/ vaporization, relate energy changes associated with a chemical reaction to the enthalpy of the reaction, and relate energy changes to PΔV work. The student is able to design and/or interpret the results of an experiment in which calorimetry is used to determine the change in enthalpy of a chemical process (heating/cooling, phase transition, or chemical reaction) at constant pressure. Science Practices 1.4 The student can use representations and models to analyze situations or solve problems qualitatively and quantitatively. 2.2 The student can apply mathematical routines to quantities that describe natural phenomena. 4.2 The student can design a plan for collecting data to answer a particular scientific question. 5.3 The student can evaluate the evidence provided by data sets in relation to a particular scientific question. Lab Hints • Enough materials are provided in this kit for 24 students working in pairs, or for 12 groups of students. For best results, schedule two 50-minute class periods for this guided-inquiry lab. An additional lab period would be needed for students to complete an optional inquiry investigation (see Opportunities for Inquiry). The pre-laboratory assignment may be completed before coming to lab, and the results and analysis may be completed the day after the lab or as homework. Students may find the MSDS from the Flinn Scientific website, www.flinnsci.com. • The best thermometers to use are digital electronic thermometers (Flinn Scientific Catalog No. AP8716) or temperature sensors connected to a computer-based interface system such as LabPro. Digital thermometers are reasonably inexpensive, update every second, and are precise to the nearest 0.1 °C. Temperature measurements may be a significant source of error in calorimetry experiments. • Two polystyrene cups nested together provide better insulation and thermal stability than one cup. If two cups are used, students can easily run two trials without rinsing and drying the cup between trials. Simply have the students interchange the actual solution cup and the bottom cup between measurements. Teaching Tips • Students may need help with the calorimetry calculations. It is important to keep track of the signs associated with the heat changes, and to understand the notion of the system versus the surroundings. A loss of heat is assigned a negative value, a gain of heat a positive value. If a reaction is exothermic, it releases or loses heat and q has a negative value. The same quantity of heat is absorbed by the solution and the heat change for the solution therefore has the same value but with a positive sign. • A slight error is incorporated into the calorimeter constant calibration to make the calculations simpler and the data collection less time-consuming. Please request Thermodynamics: Enthalpy of Reaction and Hess’s Law (Flinn Scientific Publication No. 8832) for the full calorimeter constant determination procedure. • Another type of hand warmer uses a pouch of supersaturated sodium acetate trihydrate. The crystallization of sodium acetate trihydrate from its supersaturated solution is a spontaneous physical process. The Gibbs free energy expressions for this process are: ΔG = ΔH – TΔS Equation 1 ΔG < 0 Equation 2 NaCH3CO2(aq) + 3H2O(l) → NaCH3CO2z3H2O(s) Equation 3 Because the process results in a more ordered state, –7– © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued the change in entropy, ΔS, is negative. This makes the value of –TΔS positive. Since ΔG is negative, ΔH must also be negative and its absolute value must be greater than the value of –TΔS. The crystallization reaction is highly exothermic. Depressing a metal button in the solution pouch essentially starts a chain reaction that causes the entire solution to crystallize. The liquid becomes a solid and releases so much heat that it “freezes”! This hand warmer is available from Flinn Scientific, Catalog No. AP1933. • There are two challenging conceptual hurdles students must overcome in thermochemistry—the difference between heat and temperature and the definition of the system versus the surroundings. For a reaction taking place in solution, students must realize that the liquid, the solvent, is not directly involved in the reaction. It is part of the medium, the surroundings. Reactions are generally classified as exothermic or endothermic based on the temperature change in the surroundings, which is opposite in sign to that of the system. Thus, if the temperature of the surroundings increases, it is because the energy of the system has decreased. The temperature of the system itself is often inaccessible. • One of the more stubborn student misconceptions is the idea that if the reaction mixture gets cold, it must have lost heat, therefore the reaction must be exothermic. This misconception may be traced to a lack of understanding of the system versus the surroundings. The temperature change that is measured in a typical coffee-cup calorimeter experiment is that of the surroundings. A heat of solution experiment is probably more confusing on this point than a heat of neutralization or heat of combustion experiment, because water is involved in the reaction. Also, using the combined mass of the solute and the solvent in the heat equation to calculate the heat change tends to blur the traditional distinction between the reactants and products versus the solvent. • Enthalpy is an abstract concept that is often difficult for students to understand. Most textbooks include diagrams of enthalpy versus “reaction coordinate” (reactants and products) that help students visualize the difference in the sign of ΔH for exothermic and endothermic reactions. Qualitative laboratory activities such as “Thermodynamics in a Bag” (Flinn Catalog No. AP4779) and “Discovering Instant Cold Packs” (Flinn Catalog No. AP6375) are very helpful in teaching enthalpy because they allow students to see and feel the real effects of enthalpy changes. • The most important element for success in an inquiry-based activity is student preparation. Sufficient time should be alotted for students to think through the measurements that must be made, how they will be made, the variables that will influence the measurements, and how the variables can be controlled, if necessary. Answers to Pre-Lab Questions (Student answers will vary.) 1. When chromium chloride, CrCl2, is dissolved in water, the temperature of the water decreases. (a) Is the heat of solution exothermic or endothermic? (b) Which is stronger—the attractive forces between water molecules and chromium and chloride ions, or the combined ionic bond strength of CrCl2 and intermolecular forces between water molecules? Explain. a. The heat of solution is endothermic—absorbs heat from the surroundings. b. The energy released in the formation of hydrated ions is less than the energy required to break the ionic crystal lattice and intermolecular forces between water molecules. 2. A solution was formed by combining 25.0 g of solid A with 60.0 mL of distilled water, with the water initially at 21.4 °C. The final temperature of the solution was 25.3 °C. Calculate the heat released as the solid dissolved, qsoln, assuming no heat loss to the calorimeter (see Equation 1). qsoln = –(mzszΔT) = –(25.0 + 60.0)g × 4.18 J/gz°C × (25.3 – 21.4)°C = –(85.0 g × 4.18 J/gz°C × 3.9 °C) = –1390 J 3. In Question 2 above, the calorimeter was found to have a heat capacity of 8.20 J/°C. If a correction is included to account for the heat absorbed by the calorimeter, what is the heat of solution, qsoln? qsoln = –(mzszT + Ccal ΔT) = 1390 J + [8.20 J/°C × (25.3 – 21.4)°C] qsoln = –(1390 J + 32 J) = –1420 J 4. The solid in Question 2 was aluminum sulfate, Al2(SO4)3. Calculate the molar heat of solution, ΔHsoln, for aluminum sulfate. Hint: The units for molar heat of solution are kilojoules per mole (kJ/mole). First determine the heat released per gram of solid. ΔHsoln = –(1420 J/25.0 g) × 342.15 g/mol = –19400 J/mol = –19.4 kJ/mol –8– © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued Sample Data, Results, and Analysis Part A. Heat Capacity of the Calorimeter Volume of Deionized Water, Cold 100 mL Temperature, Cold Water 22.5 °C Volume of Deionized Water, Hot 100 mL Temperature, Hot Water 47.6 °C Final Temperature 34.8 °C Temperature Change, Cold Water 12.3 °C Temperature Change, Hot Water –12.8 °C Enthalpy Change, Cold Water, qcold(J) 5146 J Enthalpy Change, Hot Water, qhot(J) –5356 J Temperature Change, Calorimeter 12.3 °C Enthalpy Change, Calorimeter, qcal(J) 209 J Calorimeter Constant, (J/°C) 17 (J/°C) Calorimeter Constant Calculation qcalor Ccal = —————— (T mix – Tinitial) Tavg = (TH+ TC)/ 2 = (47.6 + 22.5)°C/2 = (70.1/2)°C = 35.05 °C Tinitial = 22.5 °C qcalor = –qwater = (grams of water) × (specific heat of water) × (Tmix – Tavg) qcalor = –200 g × 4.18 J/gz°C × (–0.25)°C qcalor = 209 J qcalor Ccal = —————— = 209J/(34.8 – 22.5)°C = 17 J/°C (Tmix – Tinitial) Part B. Calorimetry Procedure Magnesium Sulfate, Anhydrous Volume of Deionized Water 100 mL Mass of MgSO4 5.00 g Initial Temperature 21.4 °C Final Temperature 29.3 °C Temperature Change 7.9 °C Molar Heat of Solution of Magnesium Sulfate qsoln Molar heat of solution = —————— × Molar Mass grams solute ( ) where qsoln = –(qaq + qcal) – ([105 g)(4.18 J/gz°C)(7.9 °C)] + [(17 J/°C)(7.9 °C)] 120.39 g –(3467 J + 134 J) 120.39 g Molar heat of soln = –—————————————————— × ——— = –—————— × ———– = –86.7 kJ/mole 5.0 grams mole 5.0 grams mole –9– © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued Guided-Inquiry Design and Procedure Answers to Discussion Questions 1. Review the calorimetry procedure and answer the following questions: a. What data is needed to calculate the enthalpy change for a reaction? In order to calculate the enthalpy change for a reaction, data for the three terms involved in the heat energy equation (q = m × s × ΔT) must be known or measured. The mass (m) is the mass of the solution after the solid has dissolved. The specific heat capacity (s) is assumed to be the same as the specific heat capacity of water (4.18 J/g°C). The temperature change (ΔT) is equal to the difference between the final and initial temperatures (Tfinal – Tinitial ). Note to teachers: Assuming the specific heat capacity of the solution is the same as that of water may be a major source of error in the heat calculations. b. Identify the variables that will influence the experimental data. Some of the critical variables include: (1) the mass of the solute; (2) the volume (mass) of the solvent; (3) whether all of the solute dissolves in the solvent; (4) the heat insulating properties of the reaction container; (5) how well the reaction mixture is stirred; (6) how stable the initial temperature reading is. c. What variables should be controlled (kept constant) during the procedure? The following variables should be held constant during the procedure: the volume (mass) of the solvent; the type of reaction container that is used (two insulating foam cups nestled one inside the other will provide better insulation than one cup); continuous stirring of the reaction mixture. d.The independent variable in an experiment is the variable that is changed by the experimenter, while the dependent variable responds to or depends on the changes in the independent variable. Name the independent and dependent variables in the calorimetry procedure. In a calorimetry experiment, the mass of the solute in grams is the independent variable and will be varied in different trials. The temperature change that is produced depends on the mass of the solute and is thus the dependent variable in a calorimetry experiment. e. Discuss the factors that will affect the precision of the experimental results. Many factors will influence the precision of the results: • The precision of the balance used to measure the mass of solute. • The precision of the graduated cylinder used to measure the volume of solvent. • The precision of the thermometer used to measure the temperature of the reaction mixture. • The number of times the experiment is repeated to average the effects of random errors. • The type of vessel that is used as the calorimeter—how much heat is gained or lost by the calorimeter itself. The first three measurements should be made with the most precise glassware and equipment available in the lab— centigram balances (at least), appropriate size graduated cylinders, and digital thermometers, if possible. One important way to improve the precision of the experimental results is to average data obtained over several runs or trials. A minimum of 2–3 trials is recommended. Alternatively, class data may be averaged to eliminate outlying results. – 10 – © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued Sample Data Results and Analysis Guided Inquiry Activity 45 mL Calcium Chloride, CaCl2 Volume of Deionized Water 45 mL Mass of NaCl 5.038 g Mass of CaCl2 5.075 g Initial Temperature 23.3 °C Initial Temperature 22.2 °C Final Temperature 21.9 °C Final Temperature 36.4 °C Temperature Change –1.4 °C Temperature Change 14.2 °C Sodium Acetate, NaCH3CO2 Volume of Deionized Water 45 mL Sodium Carbonate, Na2CO3 Volume of Deionized Water 45 mL Mass of NaCH3CO2 5.118 g Mass of Na2CO3 4.061 g Initial Temperature 23.4 °C Initial Temperature 22.9 °C Final Temperature 28.1 °C Final Temperature 27.9 °C Temperature Change 4.7 °C Temperature Change 5.0 °C 45 mL Ammonium Nitrate, NH4NO3 Volume of Deionized Water 45 mL Mass of LiCl 5.001 g Mass of NH4NO3 5.000 g Initial Temperature 23.7 °C Initial Temperature 23.7 °C Final Temperature 42.9 °C Final Temperature 15.7 °C Temperature Change 19.2 °C Temperature Change –8.0 °C Sodium Chloride, NaCl Volume of Deionized Water Lithium Chloride, LiCl Volume of Deionized Water Sample Calculation qsoln = –(qaq + qcal) qsoln = –(mCΔT +CcalΔT) For CaCl2qsoln = –[(50.075 g)(4.18 J/gz°C)(14.2 °C) + (17 J/°C) (14.2 °C)] qsoln = –[2972 + 241]J qsoln = –3213 J Temp. Change, ΔT, °C Calorimeter contents energy change, qaq,( J) Calorimeter energy change, qcal,( J) Internal Energy Change, qsoln, ( J) NaCl –1.4 –300 –24 324 CaCl2 14.2 2972 241 –3213 NaCH3CO2 4.7 986 80 –1065 Na2CO3 5.0 1026 85 –1111 LiCl 19.2 4017 327 –4343 NH4NO3 –8.0 –1674 –136 1810 Solid – 11 – © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued 4. Extrapolating from the information collected, predict which solid(s) could be used in an effective hand warmer meeting the following requirements: • The hand warmer must contain 10 g of an ionic solid and an inner pouch filled with 40 mL of water. • Activating the hand warmer must increase the temperature of the resulting solution by at least 20 °C. Calculations—Find the temperature change for each solid when 10 grams combine with 40 mL of water. ΔT, °C 45 mL—Observed temperature change with 45 mL of water and experimental mass of solid. ΔT, °C 40 mL—Estimated temperature change with 40 mL of water and experimental mass of solid. 10 g/40 mL ΔT—Predicted temperature increase for hand warmer containing 40 mL of water and 10 g of solid. = (10 g/ experimental mass of solid) × (ΔT, °C 40 mL) for CaCl2 = (10/5.075) × 16.2°C = 31.9°C = 45/40 × (ΔT, °C 45 mL); for CaCl2 = (45/40) × 14.4 °C = 16.2 °C Solid Cost($)/g ΔT, °C (45 mL) ΔT, °C (40 mL) 10 g/40 mL ΔT Total Cost of Solid NaCl 0.0079 –1.4 –1.6 N/A N/A CaCl2 0.0131 14.4 16.2 31.9 0.131 NaCH3CO2 0.0258 4.7 5.3 10.3 0.258 Na2CO3 0.0123 5.0 5.6 13.9 0.123 LiCl 0.0655 19.2 21.6 43.2 0.655 NH4NO3 0.0181 –8.0 –9.0 N/A N/A The best all-around hand warmer would contain calcium chloride. It produces the required temperature change and is less expensive and less toxic than LiCl. Answers to AP Chemistry Review Questions Review the following data from a calorimetry experiment to determine the heat of fusion of ice. After shaking off any excess water, several ice cubes were added to 99 g of warm water contained in a calorimeter. The initial temperature of the warm water was 46.8 °C. The ice−water mixture was stirred until the temperature reached a stable, minimum value, which was 1.1 °C. Any unmelted ice remaining at this point was immediately and carefully removed using tongs and the mass of the water in the calorimeter was measured—154 g. 1. Use the heat energy equation to calculate the amount of heat in joules released by the warm water as it cooled. ΔT = Tfinal – Tinitial = 1.1 – 46.8 °C = –45.7 °C q(warm water) = (4.18 J/g°C) × 99 g × (–45.7 °C) = –18,900 J 2. Based on the law of conservation of energy, what amount of heat was absorbed by the ice as it melted? q(ice) = –q(warm water) = +18,900 J 3. Determine the amount of energy absorbed per gram of ice as it melted. Mass of ice melted = 154 g – 99 g = 55 g Amount of energy absorbed per gram of ice as it melted = q(ice)/mass of ice = 18,900 J/55 g = 340 J/g 4. Calculate the heat of fusion (the heat required to melt ice) in units of kilojoules/mole. Heat of fusion (kJ/mole) = 340 J/g × 18 g/mole × 1 kJ/1000 J = 6.1 kJ/mole – 12 – © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654 Teacher’s Notes continued 5. The literature value for the heat of fusion of ice is 6.02 kJ/mole. What is the percent error for the experimentally determined heat of fusion? | 6.1 – 6.02 | Percent error = ——————– × 100% = 2% 6.02 6. When a mixture of ice and water originally at 0 °C is heated, the temperature remains constant (within a few degrees Celsius) until all of the ice melts. Explain what happens to the heat energy that is absorbed during this time while the temperature does not change. The energy absorbed breaks down the attractive forces that hold the water molecules in the rigid structure of ice. Reference AP* Chemistry Guided-Inquiry Experiments: Applying the Science Practices; The College Board: New York, NY, 2013. Designing a Hand Warmer—Advanced Inquiry Lab and supporting supplies are available from Flinn Scientific, Inc. Catalog No. AP7654 Description Designing a Hand Warmer—Advanced Inquiry Lab Consult your Flinn Scientific Catalog/Reference Manual for current prices. – 13 – © 2013 Flinn Scientific, Inc. All Rights Reserved. IN7654