mr4iE

https://www.youtube.com/watch?v=wwFrRy mr4iE
• batteries
• containers of chemicals waiting to be converted to electricity
• the chemical reaction does not take place until the two reactions are connected (you turn it on)
• typically create 1.5 to 9 volts
McGraw Hill Flash animation
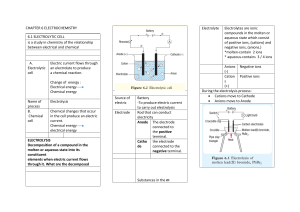
• the oxidation and reduction reactions are separated
• electrodes (two different strips of metals) are placed in each electrolyte (solution that conducts electricity)
• electrons move through a wire connecting the two electrodes
• salt bridge connects the reactions
• chemical energy changes electrical to energy through a spontaneous redox reaction
• greater the difference in reactivity of metals means greater the voltage
• the more reactive metal (higher on the activity series) is the negative electrode
(anode)
• oxidation occurs here causing loss of e-
• ex) Zn (s) Zn 2+ (aq) + 2e -
• the less reactive metal (lower on the activity series) is the positive electrode (cathode)
• reduction occurs here causing the gain of e-
• ex) Cu 2+ (aq) + 2e Cu (s)
• ions move through a salt bridge connecting the two electrolyte solutions to keep the ions created from building up in either of the solutions