INQUIRY LAB: OSMOSIS Background Information: Scientists __________________________________ Date __________
advertisement

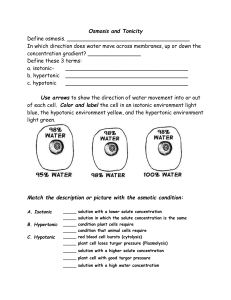
INQUIRY LAB: OSMOSIS Scientists __________________________________ Date __________ Background Information: Cells must move materials through membranes and throughout cytoplasm in order to maintain homeostasis. The movement is regulated because cellular membranes are selectively permeable. It regulates what enters and leaves the cell. Membranes are made of phospholipid bilayers containing embedded proteins. The cellular environment is aqueous. Water is a solvent in which the solutes, such as salts and organic molecules are dissolved. Water may pass through the membrane by osmosis or through specialized protein channels called aquaporins. Most other substances, such as ions, move through protein channels, while larger molecules, including carbohydrates, move through transport proteins. The simplest form of movement is diffusion, in which solutes move from an area of high concentration to an area of low concentration. Diffusion does not require energy input by cells. The movement of a solute from an area of low concentration to an area of high concentration requires energy input in the form of ATP and protein carriers called pumps. Water moves through membranes by diffusion; the movement of water through membranes is called osmosis. In a solution, water molecules surround the solute molecules. More the solute less is the concentration of free water. Water moves from areas of “high water potential” (high free water concentration and low solute concentration) to areas of “low water potential” (low free water concentration and high solute concentration). The movement of water into and out of a cell is affected by solute concentration on either side of the plasma membrane. The terms hypertonic, hypotonic, and isotonic are used to describe solutions separated by selectively permeable membranes. A hypertonic solution has a higher solute concentration and a lower water potential as compared to the solution on the other side of the membrane; therefore, water will move from hypotonic solution into the hypertonic solution through the membrane by osmosis. A hypotonic solution has a lower solute concentration and a higher water potential as compared to the solution on the other side of the membrane; therefore water will move from hypotonic solution into the hypertonic solution through the membrane by osmosis. Isotonic solutions have equal water potentials, hence water moves in both directions. A dynamic equilibrium is reached and net water movement ceases. In non-walled cells, such as animal cells, the movement of water into and out of a cell is affected by the relative solute concentration on either side of the plasma membrane. As water moves out of the cell, the cell shrinks; if water moves into the cell, it swells and may eventually burst. In walled cells, including fungal and plant cells, osmosis is affected not only by the solute concentration, but also by the resistance to water movement into the cell by the cell wall. This resistance is called turgor pressure. The presence of a cell wall prevents the cells from bursting as water enters; however, pressure builds up inside the cell and counteracts the diffusion of water into the cell. Water movement in plants is important in water transport from the roots into the shoots and leaves, driven by evaporation from the leaf which is called transpiration. Hypertonic solution Water moves from the cell into the solution Cell shrinks Crenation in animal cells Plasmolysis in plant cells Hypotonic solution Water moves from solution into the cell Cell Swells and bursts open – cytolysis Prelab: Answer the following review questions in complete sentences. 1. Explain the structure of the cell membrane (fluid mosaic model) that is responsible for its function, selective permeability. 2. Explain osmosis and how concentration of solute affects diffusion. 3. Do you think osmosis occurs when a cell is in an isotonic solution? Explain why or why not? 4. What happens to animal cells when they are placed in distilled water? 5. What prevents the plant cells from bursting when they were placed in the distilled water? 6. What kind of solution must blood be if red blood cells are to remain healthy? 7. Explain how rinsing your mouth with salt water may help to reduce swelling of the gums. (Use appropriate scientific terms: osmosis, hypotonic, hypertonic or isotonic). 8. Explain how you could revive a wilted flower (Use appropriate scientific terms: osmosis, hypotonic, hypertonic or isotonic). 9. Roads are sometimes salted to melt ice. What does this do to plants around the roadside and why? Part 1: Practice Trial Diffusion and Osmosis In this experiment, you will create models of living cells using dialysis tubing. Like cell membranes, dialysis tubing is made from a material that is selectively permeable to water and some solutes. You will fill your model cells with different solutions and determine the rate of diffusion. Step 1 Make two dialysis tubing cells by tying a knot in one end of two pieces of dialysis tubing. Step 2 Fill each “cell” with 10 mL of one of the following solutions: I. 15% sucrose II. 30% sucrose Step 3 Knot the other end, leaving enough space for water to diffuse into the cell Step 4 Create a proper data table for initial mass, final mass, and percent change of mass for each cell. Step 4 Mass each cell, record the initial mass, and then place it into a cup filled with water. Step 5 Mass the cell after 30 minutes and record the final mass. Calculate the percent change in mass using the formula: 100 x (final – initial) / initial and put it in the data table Create Data table here: Analysis questions: 1. Which solute concentration had the higher change in mass? Which one had the least change in mass? 2. Is 30% sucrose solution more or less hypertonic than a 15 % sucrose solution? (Look at your class data. Did you get twice as much reading for 30% Sucrose than 15% sucrose?) Part 2: In the hospital, patients need intravenous fluids. The intravenous fluid (IV) bag has solutes (NaCl) in water. How would you determine the best concentration of solutes to give a patient in need of fluids? Based on what you learned from part 1, design an experiment to determine the solute concentration inside a living cell? A. Model: Create a mind map or model of how different concentrations of solute affect the osmosis and movement of water in and out of cell. You will conduct an experiment to find the correct IV fluid to give a cell. Note: The patient cell that you will receive is in healthy condition which you need to maintain. All cells have some solutes. 1. What will happen if you put the cell in an IV fluid that is hypertonic to the cell? 2. What will happen if you put the cell in an IV fluid that is hypotonic to the cell? 3. What will happen if you put the cell in an IV fluid that is isotonic to the cell? 4. Which solution do you think would be best for red blood cells in the patient’s blood? To determine the correct concentration of NaCl in fluid, the class will test several solute concentartions to find the best one. Each group will test 2 solute concentrations and use the class data to find the answer. Below are the procedures. PROCEDURES 1. 1. Your group will make 2 patient cells using dialysis bags (a predetermined concentration of NaCl that is unknown to you will be provided). 2. 2. Use a graduated cylinder to measure 10mL to make each patient cell. 3. 3. Sign up for any 2 IV fluid/NaCl concentrations (0%, 0.3%, 0.6%, 0.9%, 1.2%, 1.5%) per group in the class data table. 4. 4. Write your name and one of the two concentrations on masking tape. Put it on the first cup. Write your name and another concentration on masking tape. Put it on the second cup. 5. 5.Pour IV fluid from bottles (check the on the bottle and your cup) to fill the cup about 1/3 of the way. 6. 6. Record initial mass of the patient cells in data table and on the cup that it is going in (it needs to be completely immersed in the solution). 7. 7. Keep it immersed for 30 mins. 8. 8. Record final mass of each patient cell. 9. 9. Calculate the percent change in mass using the formula: 100 x (final – initial) / initial 10. Enter percent change in mass into class data table. 11. Graph the class data for percent change of mass. There might be some negative readings. Look at the data carefully before creating the y-axis. Draw a best fit line. Create a flow chart for the experiment: Exp group: What goes in the “cell” What IV fluid goes in the beaker 1 2 3 4 Patient fluid NaCl 0% NaCl NaCl Measurement ____ g Time 30 mins Measurement ______ g B. Complete the rest of the experimental design. RESEARCH QUESTION C. After you get approval from the teacher, write the hypotheses: Null hypothesis (factor will not change rate): Alternative hypothesis ( what concentration is isotonic with patient cell ): . Get approval again before going on. 5 6 7 EXPERIMENTAL DESIGN INDEPENDENT VARIABLE: Experimental groups Control group (choose one of the experimental groups for the control): DEPENDENT VARIABLE : (what is measured, how, units): CONSTANTS: Conduct the experiment. DATA TABLES (proper data tables) Group raw data table % solution initial mass (g) final mass (g) % change mass Class average data table (% change mass) Graph the class data for percent change of mass. There might be some negative readings. Look at the data carefully before creating the y-axis. Draw a line graph and draw a best fit line. 40 30 20 10 0 -10 0 -20 2 4 6 Example: correct best fit line 8 40 30 20 10 0 -10 0 -20 2 4 6 Example: incorrect best fit line 8 Graph the class data for percent change of mass. There might be some negative readings. Look at the data carefully before creating the y-axis. GRAPH 1: (add title here) RESULTS Restate the hypothesis. State the data in words and numbers. Is alternative hypothesis supported or not. CONCLUSIONS Summarize the results section. Explain these findings using your knowledge of the science. ERROR ANALYSIS Error analysis discusses what went wrong (NOT everything that could have possibly gone wrong!). Look at the data. Does something not make sense? What might have caused that? Was some factor not held constant? How would that have affected the data? FUTURE RESEARCH Future research looks at the ‘next question.’ What other experiment could be done to learn and apply your knowledge about osmosis?