K Channels + 4/12/05, MCB610
advertisement

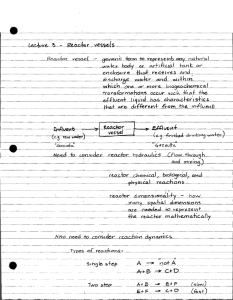
+ K Channels 60 40 mV 20 0 -20 -40 -60 -80 0 2 4 6 8 10 msec PNa or PK PNa rises quickly then declines PK rises slowly, declines with repolarization 0 2 4 6 8 10 4/12/05, MCB610 K Channel Gating K+ K+ K+ K+ K+ K+ K+ Outside Inside K+ K+ K+ K+ K+ K+ K+ -15 -60mV mV K+ K+ K+ K+ Electrophysiology – extracellular recording – intracellular recording – whole-cell recording – single channel recording How to Study? “Patch Clamp” Nobel Prize in Physiology & Medicine -1991 Extracellular Inside Cell Patch Clamp Recording Technique A B C D E el ect r ode channel cel l cel l- at t ached pat ch whol e- cel l out si de- out pat ch Types of K+ Channels Voltage-gated Inward Rectifying Ca2+ sensitive ATP-sensitive Mechano-sensitive Type A Receptor-coupled Classification of K+ Channels 1. Voltage-gated 6 transmembrane domains 4 subunits surround central pore (S5 & S6 regions of each subunit Selectivity filter (P region) – Hydrophobic sequence between last 2 TMD; contains Gly-Tyr-Gly Voltage sensor (S4) has multiple positively charged amino acids Voltage-gated con’t Activated by depolarization Present in both excitable and nonexcitable cells Functions – Regulate resting membrane potential – Control of the shape and frequency of action potentials Voltage Dependent Gating Outside S1 S2 S3 S4 S5 S6 Inside HO2C H2N + + + + + + LRVIRLVRVFRIFKLSRHS 1. Three Types Ca2+ Sensitive K+ Channels High conductance (BK) channels (Slo) – Gated by internal Ca2+ and membrane potential – Conductance = 100 to 220 picoSiemens (pS) – Blocked by charybdotoxin and iberiotoxin Intermediate conductance (IK) channels (SK4) – – – – Gated only by internal Ca2+ More sensitive than BK channels Conductance = 20 to 85 pS Blocked by charybdotoxin Small conductance (SK) channels (SK1-3) – – – – Gated only by internal Ca2+ More sensitive than BK channels Conductance = 2 to 20 pS Blocked by apamin BK channel 2. KATP channel KATP ATP increase-decrease channel opening Pancreatic type or cardiac type KNDP NDP increase-increase channel opening in the presence of Mg2+ smooth muscle type KATP characteristics Octameric four a-subunit (KIR6.1 or KIR6.2) four b-subunit (SUR1, SUR2A, SUR2B) Smooth muscle type KIR6.2/SUR2B Sulfonylurea agents-glibenclamide, tolbutamide inhibit channel activity Pharmacological KATP activator pinacidil, cromakalim, lemakalim, diazoxide, minoxidil, nicorandil (induce hyperpolarization) Endocrine Reviews 20 (2): 101-135 Molecular Biology of Adenosine TriphosphateSensitive Potassium Channels. Lydia AguilarBryan and Joseph Bryan KATP channel 3. Inwardly Rectifying K+ Channel (KIR) 2 transmembrane regions (M1 & M2) – Corresponds to S5 & S6 in Kv channel 4 subunits surround central pore P region separates M1 and M2 Non-conducting at positive membrane potentials Maintains resting membrane potential near Ek Blocked by external Ba++ Mainly Kir2x Increasing extracellular K+ induced shortening of cardiac action potential. Mg, PA 4. K2P CHANNELS TWIK: Tandem pore domain Weak Inwardly rectifying K+ channel TREK: TWIK-RElated K+ channel TRAAK:TWIK-Related Arachidonic acidActivated K+ channel TALK: TWIK-related ALkaline-activated K+ channel TASK: TWIK-related Acid-Sensitive K+ channel TREK channels NEGATIVE PRESSURE ACTIVATES SDK CHANNEL (murine colonic myocyte) A. on-cell, 0mV, asymmetrical K+ B. Pr. and Po relation -40cmH2O -60cmH2O -40cmH2O -80cmH2O -60cmH2O I-O Probability density -20cmH2O -20cmH2O 1 0.8 0.6 0.4 0.2 0 -80 -60 -40 cmH2O 10 sec 10 pA Should be K+conductance -20 0 SDK CHANNEL ACTIVATED BY INCREASE CELL LENGTH A B Stimulus of negative pressure does not necessarily stimulate the effects of cell stretch. C 10 µM -60 cm H2O Cell Elongation 2sec 10 pA Cells were actually elongated and activated K+ channels with the same properties as those activated by negative pressure. 5. A-TYPE CURRENTS IN SMOOTH MUSCLE Voltage-dependent, transient outward K+ currents have also been identified in smooth muscle cells. The term A-type current to designate rapidly activating, inactivating, voltage-dependent K+ currents. In vascular smooth muscle cells of the rabbit (portal vein, pulmonary artery, aorta), rat (pulmonary artery, renal resistance artery), and human (mesenteric artery). In genitourinary (GU) smooth muscle cells of the guinea pig (ureter, seminal vesicles, and vas deferens), rabbit (vas deferens), rat (myometrium), and human (myometrium). In gastrointestinal (GI) smooth muscle cells of the mouse (fundus, antrum, jejunum, and colon), rat (ileum), guinea pig (colon), and opossum (esophagus General properties of A-type K+ currents. A: whole cell A-type currents from holding potentials of -80 (a) and 40 mV (b) recorded from mouse antral myocytes. B: steady-state inactivation shown as a plot of normalized peak current (I/Imax) as a function of conditioning potential and fit with a Boltzmann function. Table 3. A-type channel and accessory subunit expression in smooth muscle Smooth Muscle Transcript Protein Rat mesenteric artery Kv1.4, Kv3.3, Kv3.4, Kv4.2, Kv4.3, Kv 1- 3 Rat tail artery Kv1.4, Kv3.3, Kv3.4, Kv4.2, Kv4.3, Kv 1- 3 Rat pulmonary artery Kv1.4, Kv4.1-4.3 Rat aorta Kv4.3L Rat vas deferens Kv4.3L > Kv4.2 Rat urinary bladder Kv4.3L Rat myometrium Kv4.3L > Kv4.2 Rat stomach Kv4.3L Rat colon Kv4.3L Mouse fundus Kv4.1, Kv4.2, Kv4.3L, NCS-1, KChIP1, 3, 4 Kv4.2, Kv4.3 Mouse antrum Kv4.3L > Kv4.2 > Kv4.1, NCS-1, KChIP1, 3, 4 Kv4.2, Kv4.3 Mouse jejunum Kv4.3L > Kv4.2 > Kv4.1, NCS-1, KChIP1 > KChIP2-4 Kv4.2, Kv4.3 Mouse colon Kv4.3L > Kv4.2 > Kv4.1, NCS-1, KChIP1 > KChIP2-4 Kv4.3 > Kv4. 2 Kv4.1 Kv4.3 Kv, voltage-gated Ca2+-independent K+ current; NCS, neuronal Ca2+ sensor; KChIP, K+ channel-interacting protein. Figure 1. Effect of 4-AP on the electrical activity of intact murine colonic smooth muscle Figure 2. Effect of TEA on the electrical activity of intact murine colonic smooth muscle Determination of the reversal potential Voltage dependence of inactivation and activation of delayed rectifier K+ currents The recovery from inactivation of delayed rectifier K+ current Inhibition of delayed rectifier K+ currents by 10 mM TEA Effect of 5 mM 4-AP on delayed rectifier K+ current mRNA expression of Kv1, Kv4 and Kv subunits in murine proximal colon circular smooth muscle cells The effect of intracellular Ca2+ buffering on inactivation of A-type currents The effect of KN-93 on inactivation time constants of A-type currents The effect of KN-93 on the voltage dependence of inactivation of A-type currents The effect of KN-93 on recovery from inactivation of A-type currents The effect of dialysis with autothiophosphorylated CaMKII on A-type currents CaMKII-like immunoreactivity in mouse proximal colon Quantification of Kv4 transcripts in colon and jejunum Inhibition of colonic A-type current by flecainide Kv4.2- and Kv4.3-like immunoreactivity in the tunica muscularis of murine colon and jejunum Quantification of KChIP transcripts in colon and jejunum Autothiophosphorylated Ca2+/calmodulin-dependent protein kinase II (CaMKII) decreases the rate of inactivation of voltagedependent K+ channel 4.3 (Kv4.3) currents. Autothiophosphorylated CaMKII produced a positive shift in voltage-dependent activation and inactivation. Autothiophosphorylated CaMKII accelerates the recovery from inactivation of Kv4.3 currents. Effect of mutagenesis on specific CaMKII consensus sequences on Kv4.3 currents. Effect of C2 mutagenesis on the rate of recovery from inactivation. Effect of C2 mutagenesis on Kv4.3 channel inactivation kinetics in response to application of autothiophosphorylated CaMKII Effect of C2 mutagenesis on Kv4.3 channel inactivation kinetics in response to inhibition of CaMKII. A: dialysis with the CaMKII inhibitory peptide 281–301

![Anti-Kv4.2 antibody [EP982Y] ab46797 Product datasheet 1 References 2 Images](http://s2.studylib.net/store/data/012699863_1-2dea3ba4adf27820bfb718906b682d5b-300x300.png)