Molecule Polarity and Solubility
advertisement

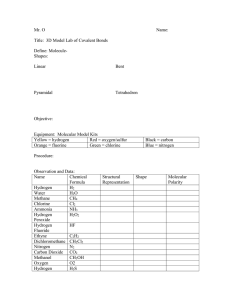
Molecule Polarity and Solubility Name: ___________________________________________ Date: ____________ Purpose: To predict whether two substances will form a solution based on the polarity of the molecules. Equipment: Molecular model building set Procedure: 1. Complete the data chart below and answer the conclusion questions by constructing molecules with the kit, using this color key: Black = Carbon Red = Oxygen Yellow = Hydrogen Green = Chlorine Blue = Nitrogen Orange = Bromine Conclusion Questions: 1. How could you tell if a molecule was polar of not? 2. How many of the molecules you built were polar? _____ 3. How many of the molecules you built were nonpolar? _____ 4. What type of molecule can dissolve in a polar solvent (polar or nonpolar)? 5. Will chlorine form a solution with ethyne? Why or why not? 6. Will carbon dioxide dissolve in ammonia? Why or why not? 7. Is hydrogen peroxide soluble in water? Why or why not? 8. Motor oil is nonpolar; which would be a better solvent to get a motor oil stain out of your clothes – water or cyclohexane? Why? 9. Iodine is a diatomic element. Knowing that, explain why you would not be able to clean up an iodine spill with water. 10. If you dissolved 10g of hydrogen bromide in 100g of methanol, which part would be considered the solute? How do you know? 1 Is it Structural Representation Symmetrical in Is it Polar? 3D? Name Formula Chlorine Cl2 Cl—Cl NH3 H—N—H | H 2 Ammonia 3 Ethyne C2H2 H—C==C—H Cl | H—C—Cl | H 4 Dichloromethane CH2Cl2 5 Nitrogen N2 N==N 6 Carbon Dioxide CO2 O==C==O H | H—O—C—H | H 7 Methanol CH3OH 8 Hydrogen Peroxide H2O2 H—O==O—H 9 Water H2O H—O—H H C 10 Cyclohexane C6H6 / HC | HC \ \ CH | CH / C H 11 Hydrogen Bromide HBr H—Br