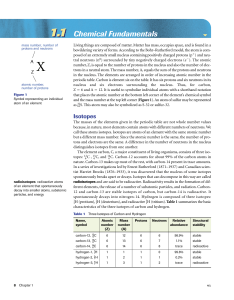
model lab
advertisement
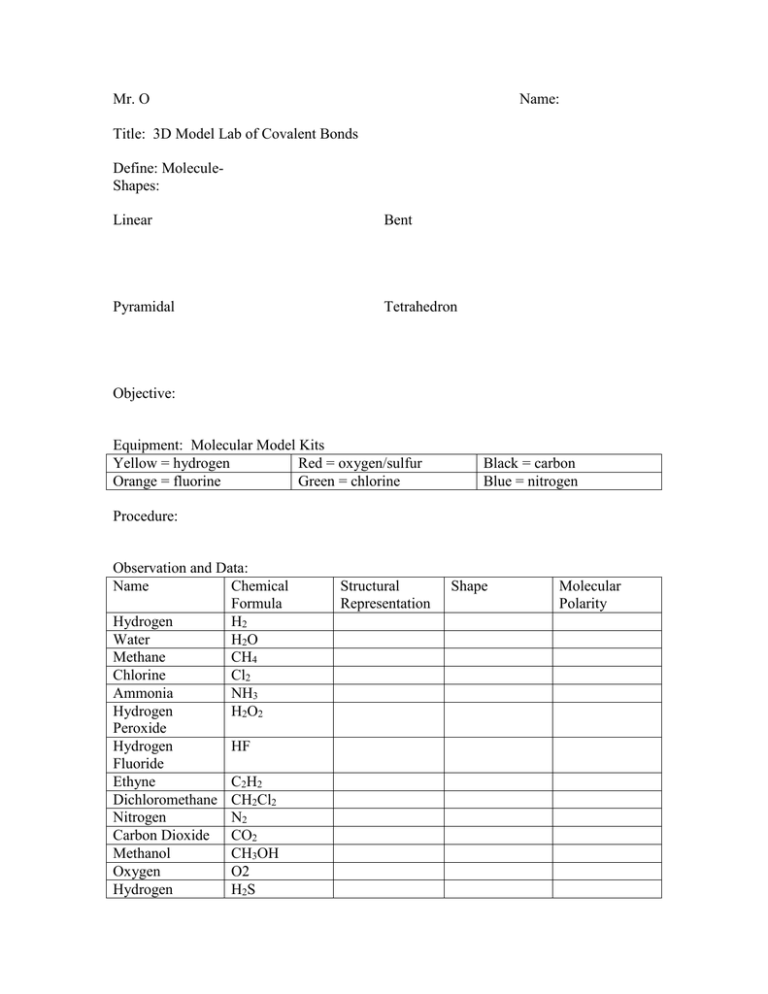
Mr. O Name: Title: 3D Model Lab of Covalent Bonds Define: MoleculeShapes: Linear Bent Pyramidal Tetrahedron Objective: Equipment: Molecular Model Kits Yellow = hydrogen Red = oxygen/sulfur Orange = fluorine Green = chlorine Black = carbon Blue = nitrogen Procedure: Observation and Data: Name Chemical Formula Hydrogen H2 Water H2O Methane CH4 Chlorine Cl2 Ammonia NH3 Hydrogen H2O2 Peroxide Hydrogen HF Fluoride Ethyne C2H2 Dichloromethane CH2Cl2 Nitrogen N2 Carbon Dioxide CO2 Methanol CH3OH Oxygen O2 Hydrogen H2S Structural Representation Shape Molecular Polarity Sulfide Propane C3H8 Conclusion Questions 1. What is the difference between a molecule and a compound? 2. Which molecules were nonpolar because all bonds were nonpolar covalent bonds? 3. Which molecules had polar covalent bonds, but were nonpolar because of symmetry? 4. Which two shapes produce polar molecules? 5. Name two types of substances that do not contain molecules with covalent bonds? 6. How many bonds can one atom of carbon make? Nitrogen? 7. Most elements want to acquire eight electrons in the outer shell, which elements do not need to follow the octet rule?