Group Activity- 4
advertisement

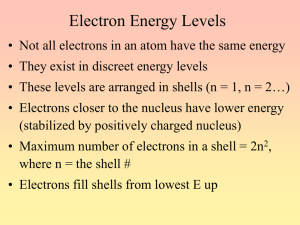
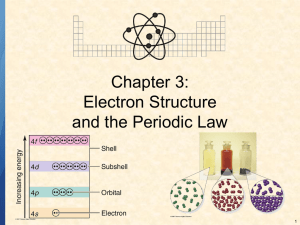
Group Activity- 4 Quantum numbers and electron configurations Name _______________________ Group Members: _________________________________ 1. State the number of electrons described by the following quantum numbers a. n = 3, l = 0 b. n = 3, l = 1 c. n = 3, l = 2, ml = -1 d. n = 5, l = 0, ml -2, ms -1/2 2. Give the n and l values for the following orbitals: a. 1s b. 2d c. 3d d. 6d e. 5p 3. How many possible orbitals are there a. n = 4 b. n = 2 c. n = 3 d. n = 5 4. Write the complete set of quantum numbers that represent the valence electrons for the following elements: a. Sodium b. Bromine 5. Fill in the blanks a. The subshell with the quantum numbers n=4, l=2 is _____________________. b. The ml values for a d orbital are ____________________________. 1 d. The allowed values of l for the shell with n=2 are ________________________________. e. The allowed values of l for the shell with n=4 are ____________________________. f. The number of orbitals in a shell with n=3 is _______________________________ g. The number of orbitals with n=3 and l=1 is _________________________________. h. The maximum number of electrons with quantum numbers with n=3 and l=2 is __________________. i. When n=2, l can be _______________________________________________. j. When n=2, the possible values for ml are ________________________________________. k. The number of electrons with n=4, l=1 is _________________________________. l. The subshell with n=3 and l=1 is designated as the ________________________ subshell. m. The lowest value of n for which a d subshell can occur is n=______________________________. Write the ground state noble gas electron configuration for each neutral atom. Indicate the outer electrons and the core electrons. 1. Na 2. Pb 3. Sr 4. U 5. N 6. Ag 7. Ti 8. Ce 9. Cl 10. Hg Write a noble gas electron configuration with equations for each ion’s formation. State whether they are diamagnetic or paramagnetic 1. Na+ 2. Pb2+ 2 3. In3+ 4. In+ 5. Mn+4 Rank the following in terms of increasing atomic radii P, N, Sr, Al, Cr, Mn, K, O Rank the following in terms of decreasing ionization energy P, N, Sr, Al, Cr, Mn, K, O Rank the following in terms of increasing ionic radii K+ O2- F- I- Rb Rb+ Rank the following in terms of increasing ionic radii Fe+ O2- Fe+2 Fe +3 3