File
advertisement
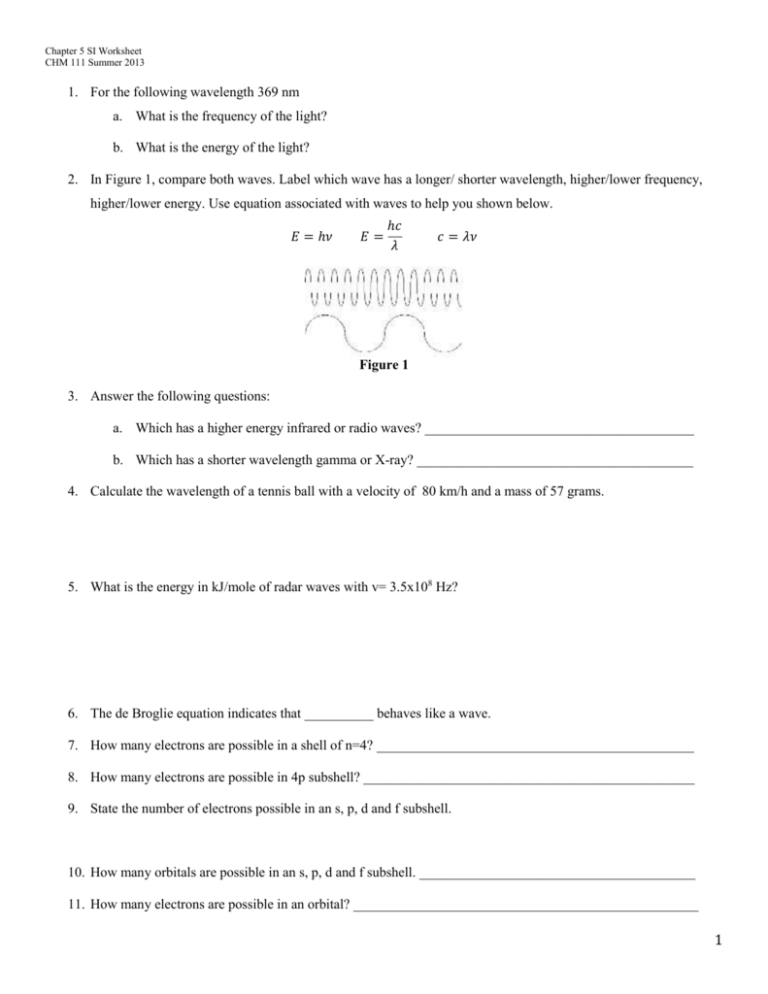
Chapter 5 SI Worksheet CHM 111 Summer 2013 1. For the following wavelength 369 nm a. What is the frequency of the light? b. What is the energy of the light? 2. In Figure 1, compare both waves. Label which wave has a longer/ shorter wavelength, higher/lower frequency, higher/lower energy. Use equation associated with waves to help you shown below. 𝐸 = ℎ𝜈 𝐸= ℎ𝑐 𝜆 𝑐 = 𝜆𝜈 Figure 1 3. Answer the following questions: a. Which has a higher energy infrared or radio waves? _______________________________________ b. Which has a shorter wavelength gamma or X-ray? ________________________________________ 4. Calculate the wavelength of a tennis ball with a velocity of 80 km/h and a mass of 57 grams. 5. What is the energy in kJ/mole of radar waves with v= 3.5x108 Hz? 6. The de Broglie equation indicates that __________ behaves like a wave. 7. How many electrons are possible in a shell of n=4? ______________________________________________ 8. How many electrons are possible in 4p subshell? ________________________________________________ 9. State the number of electrons possible in an s, p, d and f subshell. 10. How many orbitals are possible in an s, p, d and f subshell. ________________________________________ 11. How many electrons are possible in an orbital? __________________________________________________ 1 Chapter 5 SI Worksheet CHM 111 Summer 2013 12. Are the following quantum numbers possible for a given electron? a. n=4 l=4 ml=-3 ms= +1/2 b. n= 3 l=2 ml=3 ms= -1/2 c. n=3 l=1 ml= 0 ms= -1 13. Write the associated quantum numbers for a shell of n=4. 14. Define the term "node:" ____________________________________________________________________ 15. D orbitals are ______________shaped, P orbitals are _________ shaped and S orbitals are _________ shaped. 16. Match the following by writing the corresponding number next to its matching term (a,b,c). a. Pauli Exclusion Principle 1. electrons fill each orbital first before spin pairing b. Hund's Rule 2. The lowest energy fills first c. Aufbau Principle 3. No two electrons have the same 4 quantum numbers 17. In a hydrogen atom, every subshell has the same energy. What principle explains why multielectron atoms have subshells that are different in energy? 18. Define Zeff:_______________________________________________________________________________ 19. Order the following elements in order of increasing Zeff: P, Al, Na, Mg 20. How many unpaired electrons (paramagnetic) do the following ground-state atoms have? a. O c. K b. Si d. As 21. Write the electron configuration and short hand configuration for the following elements. a. O b. Ca c. Co d. I e. Pr 22. For the following ions, write the complete electron configuration a. Mg2+ b. Clc. Fe+ 23. The following configuration 1s22s23s23p64s1 has the same valance number as which of the following elements? a. Ca c. K b. Na d. Ar 2 Chapter 5 SI Worksheet CHM 111 Summer 2013 24. Write the associated quantum numbers for the following shells a. 4p b. 5d c. 3s 25. Which of the following pairs has a higher energy? a. 4s or 3p c. 6s or 4d b. 5p or 5d 26. Which of the following quantum numbers could be contained within a Z=36, Kr? a. n=3 l= 3 ml = -3 b. n=3 l=2 ml= -2 c. n=5 l=-2 ml= 0 27. Circle the element with the larger radius: a. K or Rb c. Na or Ar b. Be or F d. Fe or Os 28. Define electron affinity _______________________________________________________________________ 29. Define ionization energy ______________________________________________________________________ 30. Arrange the following elements in order of increasing ionization energy: Ne, O, F, Li 31. Arrange the following elements in order of increasing electron affinity: Ne, O, F, Li 3