Antimicrobial Medications
advertisement

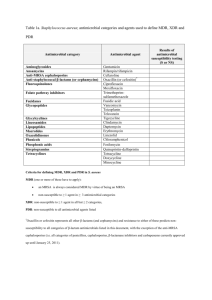
Antimicrobial Medications Antibiotics • Antimicrobial drugs naturally produced by microorganisms – Penicillium species: Penicillins – Cephalosporium specis: cephalosporins – Streptomyces species: • lincosamides, aminoglycosides, tetracyclines, chloramphenicol Features of antimicrobial drugs • Selective toxicity – Therapeutic index • Antimicrobial action – Bactericidal – Bacteristatic • Spectrum of activity – Broad spectrum – Narrow spectrum • Combination effects – Antogonistic – Synergistic – Additive Pharmacokinetics: what happens to the drug in the body? • • • • • Absorption Tissue distribution Metabolism Route of excretion Rate of elimination Adverse effects • Adverse drug reaction • Toxic effects • Suppression of normal microbiota Mechanisms of antimicrobial drugs • • • • • Inhibition of cell wall synthesis Inhibition of protein synthesis Inhibition of nucleic acid synthesis Inhibition of biosynthetic pathways Disruption of cell membrane integrity Targets of Cell Wall Synthesis Penicillin • Inhibits formation of tetrapeptide side chains How organisms degrade penicillins Family of Penicillins • • • • • Natural penicillins Penicillinase-resistant penicillins Broad-spectrum penicillins Extended-spectrum penicillins Penicillins plus beta-lactamase inhibitors Family tree of penicillins β-lactams • • • • • Penicillins Cephalosporins Carbapenems Vancomycin Bacitracin Cephalosporins • Derived from fungus, Acremonium cephalosporium • Chemical structure makes them resistant to beta-lactamase: low affinity for penicillin binding proteins • Grouped into first, second, third, and fourth generation cephalosporins Vancomycin • Binds to the terminal amino acids of the peptide chain of NAM molecules, blocks peptidoglycan formation Antibiotics that inhibit protein synthesis Oxazolidinones • Reversibly bind to the 50S subunit, interfere with initiation of protein synthesis • Used for treating gram positive infections resistant to Beta-lactam drugs and Vancomycin • Ex: Linezolid Aminoglycosides • Bactericidal • Irreversibly bind to 30S ribosome, cause misreading of the mRNA • Transported into cells that actively respire (not effective against ananerobes, streptococci, enterococci) • Ex: streptomycin, gentamicin, tobramycin Tetracyclines & Glycylcyclines • Bind reversibly to 30S, block attachment of the tRNA to ribosome • Actively transported into bacterial cells • Effective against gram positive and gram negative • Resistance: due to decrease in uptake or increase in excretion • Ex: Doxycycline Macrolides • Reversibly bind to the 50S, prevent continuation of protein synthesis • Drug of choice for patients allergic to penicillins • Not good for Enterobacteriaceae • Ex: Erythromycin, Azithromycin • Resistance: enzymes that alter drug, decreased uptake Inhibition of protein synthesis: Chloramphenicol • Rare side effect = irreversible bone marrow suppression • Banned in food animals • Making a come-back in companion animal medicine due to effectiveness against multidrug resistant staphylococci Antibiotics that inhibit nucleic acid synthesis • Fluoroquinolones – Interferes with function of topoisomerase • Rifamycins – Blocks prokaryotic RNA polymerase from initiating transcription Antibiotics that inhibit biosynthetic pathways • Sulfonamides • Trimethoprims Sulfonamides (sulfa drugs) • First synthetic drugs to treat microbial infections • Used to treat urinary tract infections (UTIs) • Combination of trimethoprim and sulfamethoxazole (TMP-SMZ) example of synergism Drugs used together inhibit folic acid synthesis Tests for microbial susceptibility • Kirby-Bauer (disk diffusion method) Tests for microbial susceptibility • Minimum Inhibitory Concentration: MIC – Grow bacteria in a serial dilution of the antimicrobial being tested – Fixed concentration of bacterial cells – Observation of turbidity after 16 hrs-24 hrs of growth – Lowest concentration of drug that inhibits growth = MIC Minimum Inhibitory Concentration • Manual broth dilution method • Automated broth dilution method • E-test Determining the Minimum Inhibitory Concentration (MIC) Automated MIC E-test for MIC Zone size & MIC values • Raw data • Meaningless without interpretation • Correlation of in vitro results with achievable levels of drug concentration in a live patient • Correlation with actual clinical outcome What resistance looks like… Mutant Prevention Concentration? Mechanisms of acquired drug resistance • Destruction or inactivation of the drug: drug inactivation enzymes • Alteration of target molecule (mutation) • Decreased uptake: alteration of porins • Increased elimination: efflux pumps Acquiring resistance • Spontaneous mutation • Gene transfer – R plasmids Emerging antimicrobial resistance Streptococcus pneumoniae • Altered penicillin binding proteins – DNA-mediated transformation Enterococcus species • Gram positive enteric cocci; facultative anaerobes; formerly classified as Group D streptococcus • Common cause of nosocomial infections – Enterococcus faecalis, Enterococcus faecium • Intrinsic resistance: • Acquired resistance Mycobacterium tuberculosis • Multidrug-resistant M. tuberculosis – Resistance to isoniazid & rifampin • Extensively drugresistant M. tuberculosis – Resistance to isoniazid & rifampin + 3 or more of the 2nd line drugs Enterococcus species: intrinsic resistance • Low affinity of penicillin binding proteins for many β-lactam antibiotics, esp. cephalosporins • Resistance to potentiated sulfonamides (i.e., trimethoprim-sulfa): able to utilize external sources of folate • Low permeability for aminoglycosides – Treatment with a cell-wall active drug such as ampicillin is synergistic (allows the drug to get into the cell) UNLESS high-level gentamicin resistance is present Enterococcus species: acquired resistance • High-level gentamicin-resistance: plasmidencoded inactivating enzymes • Tetracycine resistance: efflux pumps, ribosomal protection • Macrolide resistance: efflux pumps • Vancomycin resistance: altered drug binding site on cell wall Enterobacteriaceae • Gram negative enteric rods • Intrinsic resistance to many drugs due to outer membrane • β-lactamases: enzymatic inactivation of the lactam ring • Extended spectrum βlactamases (ESBL+) • Carbapenemresistance: enzymatic inactivation Staphylococcus species • • • • Staph aureus Staph pseudintermedius Staph schleiferi Methicillin-resistant staph: penicillinase + altered penicillin-binding proteins with low affinity for β-lactam drugs (mecA gene on R plasmid) • Vancomycin-resistant staph Methicillin-resistant staphylococci • MRSA: methicillin-resistant Staph aureus – drug resistance + increased pathgenicity • MRSP: methicillin-resistant Staph pseudintermedius – Acquisition of drug resistance is not associated with acquisition of new virulence factors • MRSS: methicillin-resistant Staph scheiferi – Drug resistance/no new virulence factors • Coagulase-negative MRS Coagulase test • Tests for coagulase enzyme = virulence factor produced by Staphylococcus aureus, Staph. pseudintermedius and Staph. schleiferi subspecies coagulans • Important in differentiating potentially pathogenic from non-pathogenic species of staphylococci Coagulase enzymes • Bound coagulase (“clumping factor”) – attached to bacterial cell wall – Coagulase enzyme + fibrinogen in plasma → fibrin clot surrounding bacteria: prevents antibody and complement binding, prevents phagocytosis, protects from NETs • Free coagulase – secreted enzyme – Coagulase enzyme + CRF → conversion of prothrombin to thrombin and fibrinogen to fibrin Coagulase slide test • Rabbit plasma + bacteria: agglutination within 1-2 minutes = positive result – Detects only bound coagulase – False negatives or equivocal results are common – Negative or equivocal tests have to be confirmed with tube test Coagulase tube test • Rabbit plasma + bacteria: coagulation of the plasma = thickening OR formation of fibrin clumps or threads – Standard practice = read at 4 hrs, if negative recheck at 24 hrs • Tests not read at 4 hrs that are negative at > 4 hrs will have to be repeated because early positive results may revert to a negative result Coagulase negative staph • Common isolates from skin cultures • Non-pathogenic commensuals • Rarely part of mixed population in deep skin/ wound infections (furuncles) • Rarely cause bacteremia or other systemic infections in immune-compromised individuals • Commonly carry plasmids with mecA gene MRS: colonization vs. infection • Sharing of plasmids + high antimicrobial use • Selecting for MRS population • Increasing % of staphylococcal isolates from non-lesional skin and nasal mucosa are methicillin resistant Responsible drug use • • • • • • Use vs misuse of antimicrobial drugs Responsibilities of health care professionals? Responsibilities of patients? Responsibilities of pet owners? Public education Over the counter antimicrobial drugs – Developing countries – US feed stores Antimicrobial Stewardship • Increasing drug resistance • Fewer drugs in development • Drugs being developed don’t have novel targets • Drugs being developed are broad spectrum • “Bad Bugs, No Drugs” task force 10x20 initiative Antimicrobial Stewardship • 4 D’s of antimicrobial therapy – Right Drug, – Right Dose, – De-escelation to pathogen directed therapy – Right Duration of therapy • Prevent overuse, misuse and abuse • Minimize the development of resistance