Gibbs free energy
advertisement
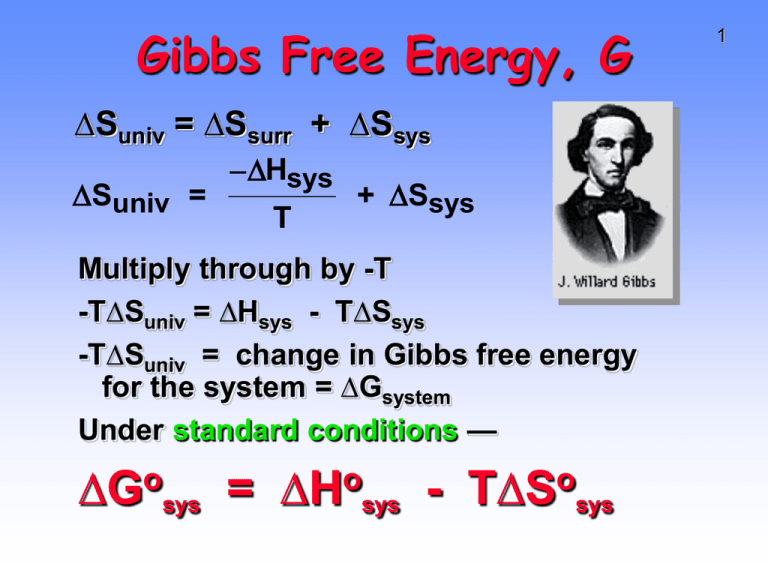
Gibbs Free Energy, G ∆Suniv = ∆Ssurr + ∆Ssys Suniv = Hsys T + Ssys Multiply through by -T -T∆Suniv = ∆Hsys - T∆Ssys -T∆Suniv = change in Gibbs free energy for the system = ∆Gsystem Under standard conditions — o ∆G sys = o ∆H sys - o T∆S sys 1 o ∆G Gibbs free = o ∆H - o T∆S 2 energy change = total energy change for system - energy lost in disordering the system If reaction is • exothermic (negative ∆ Ho) (energy dispersed) • and entropy increases (positive ∆So) (matter dispersed) • then ∆Go must be NEGATIVE • reaction is spontaneous (and productfavored). o ∆G = o ∆H - o T∆S 3 Gibbs free energy change = total energy change for system - energy lost in disordering the system If reaction is • endothermic (positive ∆Ho) • and entropy decreases (negative ∆So) • then ∆Go must be POSITIVE • reaction is not spontaneous (and is reactant- favored). Gibbs Free Energy, G o ∆G = o ∆H - o T∆S ∆Ho ∆So ∆Go Reaction exo(–) increase(+) – Prod-favored endo(+) decrease(-) + React-favored exo(–) decrease(-) ? T dependent endo(+) increase(+) ? T dependent 4 Gibbs Free Energy, G o ∆G = o ∆H - 5 o T∆S Two methods of calculating ∆Go a) Determine ∆Horxn and ∆Sorxn and use GIbbs equation. b) Use tabulated values of free energies of formation, ∆Gfo. ∆Gorxn = ∆Gfo (products) - ∆Gfo (reactants) Free Energies of Formation Note that ∆G˚f for an element = 0 6 Calculating ∆Gorxn 7 Combustion of acetylene C2H2(g) + 5/2 O2(g) --> 2 CO2(g) + H2O(g) Use enthalpies of formation to calculate ∆Horxn = -1238 kJ Use standard molar entropies to calculate ∆Sorxn = -97.4 J/K or -0.0974 kJ/K ∆Gorxn = -1238 kJ - (298 K)(-0.0974 J/K) = -1209 kJ Reaction is product-favored in spite of negative ∆Sorxn. Reaction is “enthalpy driven” Calculating ∆Gorxn NH4NO3(s) + heat ---> NH4NO3(aq) Is the dissolution of ammonium nitrate productfavored? If so, is it enthalpy- or entropy-driven? 8 Calculating ∆Gorxn 9 NH4NO3(s) + heat ---> NH4NO3(aq) From tables of thermodynamic data we find ∆Horxn = +25.7 kJ ∆Sorxn = +108.7 J/K or +0.1087 kJ/K ∆Gorxn = +25.7 kJ - (298 K)(+0.1087 J/K) = -6.7 kJ Reaction is product-favored in spite of negative ∆Horxn. Reaction is “entropy driven” Gibbs Free Energy, G o ∆G = o ∆H - 10 o T∆S Two methods of calculating ∆Go a) Determine ∆Horxn and ∆Sorxn and use GIbbs equation. b) Use tabulated values of free energies of formation, ∆Gfo. ∆Gorxn = ∆Gfo (products) - ∆Gfo (reactants) Calculating ∆Gorxn ∆Gorxn = ∆Gfo (products) - ∆Gfo (reactants) 11 Combustion of carbon C(graphite) + O2(g) --> CO2(g) ∆Gorxn = ∆Gfo(CO2) - [∆Gfo(graph) + ∆Gfo(O2)] ∆Gorxn = -394.4 kJ - [ 0 + 0] Note that free energy of formation of an element in its standard state is 0. ∆Gorxn = -394.4 kJ Reaction is product-favored as expected. Free Energy and Temperature 2 Fe2O3(s) + 3 C(s) ---> 4 Fe(s) + 3 CO2(g) ∆Horxn = +467.9 kJ ∆Sorxn = +560.3 J/K ∆Gorxn = +300.8 kJ Reaction is reactant-favored at 298 K At what T does ∆Gorxn just change from being (+) to being (-)? When ∆Gorxn = 0 = ∆Horxn - T∆Sorxn Hrxn 467.9 kJ T = = = 835.1 K Srxn 0.5603 kJ/K 12 13 More thermo? You betcha! Thermodynamics and Keq FACT: ∆Gorxn is the change in free energy when pure reactants convert COMPLETELY to pure products. FACT: Product-favored systems have Keq > 1. Therefore, both ∆G˚rxn and Keq are related to reaction favorability. 14 Thermodynamics and Keq Keq is related to reaction favorability and so to ∆Gorxn. The larger the value of K the more negative the value of ∆Gorxn o ∆G rxn = - RT lnK where R = 8.31 J/K•mol 15 Thermodynamics and Keq ∆Gorxn = - RT lnK Calculate K for the reaction N2O4 --->2 NO2 ∆Gorxn = +4.8 kJ ∆Gorxn = +4800 J = - (8.31 J/K)(298 K) ln K 4800 J lnK = = - 1.94 (8.31 J/K)(298K) K = 0.14 When ∆Gorxn > 0, then K < 1 16 ∆G, ∆G˚, and Keq • ∆G is change in free energy at nonstandard conditions. • ∆G is related to ∆G˚ • ∆G = ∆G˚ + RT ln Q where Q = reaction quotient • When Q < K or Q > K, reaction is spontaneous. • When Q = K reaction is at equilibrium • When ∆G = 0 reaction is at equilibrium • Therefore, ∆G˚ = - RT ln K 17 ∆G, ∆G˚, and Keq Figure 19.10 18 ∆G, ∆G˚, and Keq • Product favored reaction • –∆Go and K > 1 • In this case ∆Grxn is < ∆Gorxn , so state with both reactants and products present is MORE STABLE than complete conversion. 19 ∆G, ∆G˚, and Keq 20 Product-favored reaction. 2 NO2 ---> N2O4 ∆Gorxn = – 4.8 kJ Here ∆Grxn is less than ∆Gorxn , so the state with both reactants and products present is more stable than complete conversion. ∆G, ∆G˚, and Keq 21 Reactant-favored reaction. N2O4 --->2 NO2 ∆Gorxn = +4.8 kJ Here ∆Gorxn is greater than ∆Grxn , so the state with both reactants and products present is more stable than complete conversion. Thermodynamics and Keq Keq is related to reaction favorability. When ∆Gorxn < 0, reaction moves energetically “downhill” ∆Gorxn is the change in free energy when reactants convert COMPLETELY to products. 22