Summary of Chapter 18 (Thermodynamics)
advertisement

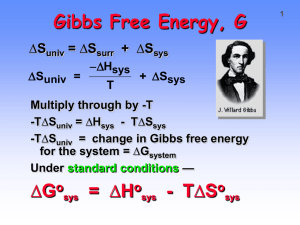
Summary of Chapter 18 (Thermodynamics) Second Law of Thermodynamics All spontaneous processes (reactions) cause an increase in the entropy of the universe. A process occurring at equilibrium has no effect on the entropy of the universe. Imagine that you are doing a reaction. At some arbitrary time, you want to know whether the reaction is at equilibrium or whether it is still proceeding. What do you do? 1. If you know the value of Keq, take a sample out of the reaction vessel, stop the reaction, and determine the value of Q at that moment. Compare Q to Keq. 2. If you don’t know the value of Keq, determine what the reaction would do to the entropy of the universe if it was to continue in the forward direction. That is, calculate Suniv (see below) and determine its sign. If Suniv > 0 then Q < Keq and the reaction will proceed (spontaneously) in the forward direction. If Suniv < 0 then Q > Keq and the reaction will proceed (spontaneously) in the reverse direction. If Suniv = 0 then Q = Keq and the reaction is at equilibrium. How do you calculate Suniv? Calculate Grxn! Suniv = Ssys + Ssurr Ssurr = Hsys/T (Note: Ssys ≠ Hsys/T) Suniv = Ssys Hsys/T Suniv = TSsys + Hsys Define Gsys = Suniv (note: GsysGrxn) Gsys = Hsys TSsys If Grxn < 0 then Suniv > 0 (The reaction will proceed in the forward direction.) If Grxn > 0 then Suniv < 0 (The reaction will proceed in the reverse direction.) If Grxn = 0 then Suniv = 0 (The reaction is at equilibrium.) Grxn is the change in “Gibbs Free Energy”. It tells you how far the reaction is from equilibrium and which direction the reaction must proceed to reach equilibrium. (This is the same as comparing Q to Keq.) Thus, it also tells you how much work the reaction can perform as it proceeds toward equilibrium. 1 OK, but how do you calculate Grxn? 1. Calculate the standard free energy change (Gorxn) using values from appendix 3 and this equation: Gosys = Hosys TSosys Gorxn is the value of Grxn when Q = 1. So it tells you which way a reaction goes when Q = 1. It also tells you the value of Keq (see below). 2. Calculate the value of Grxn for the actual value of Q using this equation: Grxn = Gorxn + RTlnQ This tells you which way a reaction goes for any value of Q. Notice that Grxn = Gorxn when Q = 1 (as expected)! How do you calculate the value of Keq from Gorxn? Grxn = Gorxn + RTlnQ When Q = Keq, Grxn = 0 (at equilibrium) so: Gorxn = -RTln Keq or Keq = e ΔG /RT o Gorxn is a special value of Grxn. It is the value of Grxn when Q = 1. Keq is a special value of Q. It is the value of Q when the reaction is at equilibrium. Both Grxn and Q tell you how far you are from equilibrium. Both Gorxn and Keq tell you where the equilibrium point is. Third law of thermodynamics Appendix 3 lists values of So for a variety of substances in their standard states at 298 K. These numbers are the absolute entropy content for one mole of each substance. The reason these numbers can be measured is because of the 3rd law: The entropy of a perfect crystal at absolute zero is zero. One can measure the entropy contained in a given amount of substance because there is a physically meaningful zero point! (See optional material for how S is measured.) 2 Other useful forms of the thermodynamic equations Equilibrium Temperature: For a given value of Q, at what temperature is Q = K. This is the equilibrium temperature, Teq, Gsys = Hsys TSsys At equilibrium, Gsys = 0 and T = Teq. Therefore: Teq = Hsys/Ssys Notice that this equation only makes sense if Hsys and Ssys have the same sign. (You can’t have a negative Kelvin temperature.) This is not an issue because reactions with opposite signs of Hsys and Ssys do not reach equilibrium. (They have extremely large or extremely small equilibrium constants.) Experimental Determination of Horxnand Sorxn: Gorxn = -RTln Keq = Hosys TSosys ln Keq = ΔH o 1 ΔS o R T R A plot of ln Keq vs. 1/T give a straight line with: slope = ΔH o ΔSo and y-intercept = R R Reactions that reach equilibrium have the same sign for Hosys and Sosys. This equation says that the equilibrium constant increases as temperature increases if both Hosys and Sosys are positive. (Raising the temperature shifts the equilibrium in the endothermic direction and toward the more disordered state!) 3