pH Lab Handout and Data
advertisement

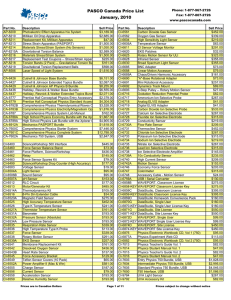
Equipment: * pH Sensor PASPORT (PS-2102) 6507A) | ScienceWorkshop (CI- * Computer Interface: PASPORT: Xplorer GLX (PS-2002) | Xplorer (PS-2000) | USB Link (PS-2100A) | PowerLink (PS2001) ScienceWorkshop: 500 Interface (CI-6400) | 750 Interface - SCSI (CI-6450) | 750 Interface USB (CI-7650) * 5 dixie cups (small beakers or plastic cups may also be used) and a Test Tube Rack. * Wash bottle filled with distilled water and large beaker (or use a sink), to rinse off the pH sensor between measurements. * Solutions: gatorade, Coca-cola black, Full Throtle, Sobe lean, milk, and Distilled Water. Back to top Equipment Setup: 1. Label each of the dixie cups with the names of the various solutions. 2. Pour approxiamtely 10 ml of each solution into the respective, labeled test tube. 3. Set wash bottle and waste beaker close by so that the pH Sensor can be easily rinsed between readings. Software Setup: 1. Click on one of the links below to download a pre-configured DataStudio file for this pH experiment, and then open the file. PASPORT users: Windows (.zip file) or Macintosh (.sit file) ScienceWorkshop 500 users: Windows (.zip file) or Macintosh (.sit file) 2. Connect the pH sensor to the USB link or Xplorer (PASPORT users), or to the 500 interface (ScienceWorkshop 500 users). Additionally, for ScienceWorkshop 500 users – associate the pH sensor with the interface in the Experiment Setup window (double-click or drag). 3. The PASPORT pH sensor typically does not need to be calibrated; its accuracy is approximately ± 0.5 pH units. If better accuracy is needed, or to calibrate the CI-6507A pH Sensor, refer to DataStudio’s online help menu for specific calibration instructions. Data Collection Procedure: 1. Place the pH Sensor into the first test tube (solution #1) so that the solution just covers the tip of the probe. 2. Click the Start button ( ) to begin collecting data. Because the preconfigured DataStudio file has been prepared for manual sampling, the Start button will change to a Keep ( ) button. 3. Watch the digits display for the pH reading to stabilize, then click the left side of the Keep button. 4. A dialog box like the one shown below will appear and allow you to enter a value for solution #. Enter "1" and click OK. Notice the graph and table will update automatically. 5. Remove the pH Sensor from the test tube and thoroughly rinse it with distilled water before measuring the next solution. 6. Place the pH Sensor into the second test tube (solution #2) and repeat the steps described above to record the second pH measurement. Continue in this manner until all ten solutions have been measured, then click the right side ("Stop" ) of the Keep button. Data Analysis: 1. Scale the axes to fit the data using the Scale to Fit button ( ) in the Graph toolbar. 2. Observe how many substances registered below 7 on the pH scale, at or near 7, or above 7. Use the data to classify the substances as acidic, basic, or neutral. Record these observations in a data table like the one shown below. Data Table: Name of substance Sobe Lean Distilled water Coca-cola black Full throttle Gatorade Milk Solution color Pink Clear pH 3.30 7.05 Acid, base, or neutral acid Neutral Brown 3.64 Acid Yellow 3.43 Acid Orange White 3.24 6.95 Acid Neutral Conclusions and Extensions: 1. Were more of the test substances acidic or basic? How can you tell? 2. Does the color of the solution determine its pH level? 3. What was the pH of distilled water? Why is this value significant? 4. A local pond had a pH reading of 6 but then it measured 4 after a recent rain storm. By what factor did its acidity increase? What likely caused the pH level change? 5. In order to increase the pH of a solution, what kind of substance (acidic, basice, neutral) must be added? 6. Why is pH important to all living things? 7. Explain the role of a buffer in a living system.