1305-Home-work-11.doc

CHEM 1305-Home-work 11 NAME:
1) For which of the following pairs of elements would “electron transfer” between atoms most likely occur?
A) Na and Cd B) Ca and O C) As and I
D) V and Li E) O and F
2) A bond where the electrons are shared unequally is called a:
A) polar covalent B) coordinate covalent
C) purely (nonpolar) covalent D) ionic
3) Which of the following is the correct Lewis structure for OF
2
?
A) F O F
B) F F O
C) F O F
D) F O F
4) The total number of “shared electron pairs” in the molecule H
2
S is ________.
A) 1 B) 2 C) 4 D) 8
5) The total number of valence electrons in a molecule of SOF
2
is
A) 26 B) 24 C) 18 D) 20
6) Indicate the total PAIRS of electrons which would be shown as “dots” in a correctly written Lewis
structure for OF
2
.
A) 9 B)16 C) 13 D) 11 E) 10
7) The Lewis structure for the SO
4
2
ion contains how many “bonding electrons PAIRS?”
A) 2 B) 4 C) 8 D) 12 E) 6
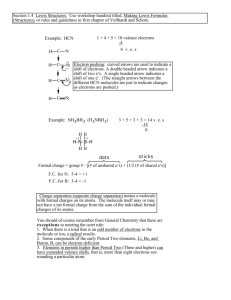
8) In the Lewis structure of HCN, how many nonbonded electron pairs are present?
A) 3 B) 4 C) 5 D) 2 E) 1
9) The geometry associated with three pairs of bonded electrons and one pair of nonbonded electrons about a central atom in a molecule is ________.
A) tetrahedral B) trigonal pyramidal C) trigonal planar
D) angular E) linear
10) The molecular shape (geometry) of a NCl
3
molecule is ________.
A) linear B) angular C) trigonal planar
D) tetrahedral E) trigonal pyramidal
11) What is the total number of valence electrons in one molecule of N2O3?
A) 2 B) 11 C) 18 D) 28 E) none of the above
12) Draw the electron dot formula for silicon tetrabromide, Si Br4. How many pairs of nonbonding
electrons are in a silicon tetrabromide molecule?
A) 1 B) 2 C) 4 D) 12 E) none of the above
13) Which of the following is a general trend for the electronegativity of elements in the periodic table?
A) increases from left to right; increases from bottom to top
B) increases from left to right; decreases from bottom to top
C) decreases from left to right; increases from bottom to top
D) decreases from left to right; decreases from bottom to top
E) none of the above
14) Which of the following shows a general increasing trend for electronegativity in the periodic table?
A) down a group of metals
B) down a group of nonmetals
C) across a series of metals from right to left
D) across a series of nonmetals from right to left
E) none of the above
15) According to the general trends in the periodic table, which of the following is the most electronegative element?
A) Ar B) Cl C) S D) P E) Si
16) Given the electronegativity values of C (2.5), N (3.0), O (3.5), and S (2.5), which of the following molecules have polar bonds?
A) carbon dioxide, CO2
B) sulfur dioxide, SO2
C) smog, NO2
D) all of the above
E) none of the above
17) Given the electronegativity values of C (2.5) and O (3.5), illustrate the bond polarity in a carbon monoxide molecule, CO, using delta notation.
A) (δ+) C–O (δ+)
B) (δ+) C–O (δ-)
C) (δ-) C–O (δ+)
D) (δ-) C–O (δ-)
E) none of the above
18) Given the values for Br (2.8) and F (4.0), calculate the electronegativity difference in a bromine monofluoride bond, BrF.
A) 1.2
B) 6.8
C) -1.2
D) -6.8
E) none of the above
19) Which of the following occurs naturally as nonpolar diatomic molecules?
A) argon
B) chlorine
C) sulfur
D) all of the above
E) none of the above
20) What is the electron pair geometry for a phosphine molecule, PH3?
A) bent
B) linear
C) tetrahedral
D) trigonal pyramidal
E) none of the above