Chapter 18 The Nucleus: A Chemist’s View
advertisement
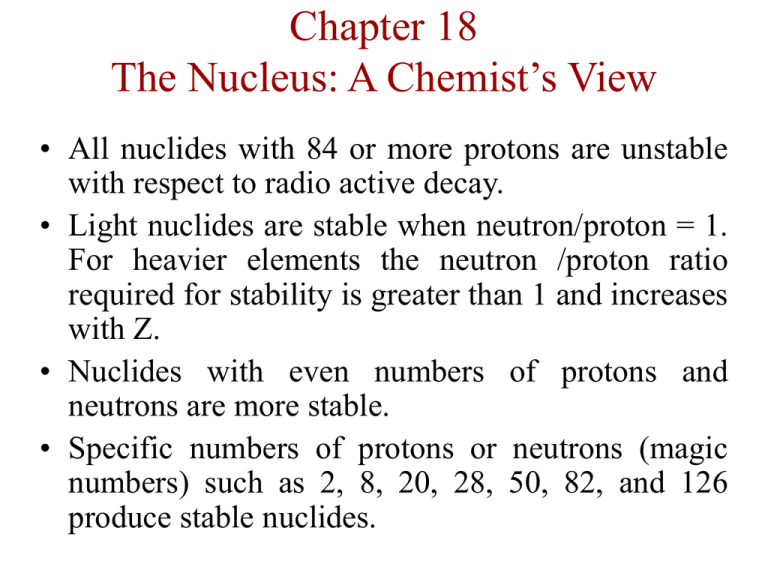
Chapter 18 The Nucleus: A Chemist’s View • All nuclides with 84 or more protons are unstable with respect to radio active decay. • Light nuclides are stable when neutron/proton = 1. For heavier elements the neutron /proton ratio required for stability is greater than 1 and increases with Z. • Nuclides with even numbers of protons and neutrons are more stable. • Specific numbers of protons or neutrons (magic numbers) such as 2, 8, 20, 28, 50, 82, and 126 produce stable nuclides. Figure 18.1 The Zone of Stability Types of Radioactive Decay A nucleus will undergo decomposition to form a different nucleus which is known as radioactive decay. 4 Alpha production (): helium nucleus, 2 He 238 4 92 U 2 He 234 90Th 0 Beta production (): 1 e (mass number remains constant). Net effect is to change a neutron to a proton. 234 234 0 90Th (thorium) 91Pa 1e (protactinium) Types of Radioactive Decay Gamma ray production (): (high energy photon) 238 4 92 U 2 He 234 90Th 0 20 Positron production: (particle with same mass as the electron but net effect is to change a proton to a neutron. 22 0 11 Na 1e 22 10 Ne Electron capture: (inner-orbital electron is captured by the nucleus) 201 0 201 Hg e 80 1 79 Au 00 Decay Series Sometimes a radioactive nucleus cannot reach a stable state through a single decay process. A radioactive nucleus reaches a stable state by a series of steps. 232 series of decays 208 Th Pb 90 82 Figure 18.2 A Decay Series Rate of Decay Rate of decay is the negative of the change in the number of nuclides per unit time, rate = -(N/t)N Rate = -N/ t = kN (k = proportionality constant) The rate of decay is proportional to the number of nuclides. This represents a first-order process. Integrated first-order rate law is: ln(N/No) = -kt where, No = original number of nuclides (at t = 0) and N = number remaining at time t. Half-Life . . . the time required for the number of nuclides to reach half the original value (N0/2). When, t = t1/2, N = No/2 ln(N/No) = -kt ln[(No/2)/No] = -kt1/2 t1/ 2 ln(2) 0.693 k k (half life is constant) If the half-life of a radioactive nuclide is known, the rate constant can be calculated. Figure 18.3 The Decay of a 10.0g Sample of Strontium-90 Over Time Nuclear Transformation The change of one element into another. 27 4 30 1 13 Al 2 He 15 P 0 n 249 Cf 98 18 263 1 O X 4 n 8 0 106 Figure 18.5 A Schematic Diagram of a Cyclotron Figure 18.6 A Schematic Diagram of a Linear Accelerator Detection of Radioactivity Geiger-Muler Counter: High energy particle from radioactive decay processes produce ions when they travel through matter. The probe of the Geiger counter is filled with Ar gas which can be ionized by a rapidly moving particle. high energy Ar(g) Ar+(g) + eparticle Electric device detect the current flow and the number of events can be counted. Thus the decay rate of the radioactive sample can be determined. Figure 18.7 A Schematic Representation of a Geiger-Müller Counter Detection of Radioactivity Scintillation Counter: Takes the advantage of the fact that certain substances, such as zinc sulfide, gives off light when they are struck by high energy radiation. A photocell senses the flashes of light that occur as the radiation strikes and thus measures the number of decay events per unit of time. Energy and Mass When a system gains or loses energy it also gains or loses a quantity of mass. E = mc2 E 2 m c m = mass defect E = change in energy If E = (exothermic), mass is lost from the system. Binding Energy . . .is the energy required to decompose the nucleus into its components. 56 Iron-56 26 Fe is the most stable nucleus, which has a binding energy per nucleon of 8.79 MeV. Figure 18.9 The Binding Energy Per Nucleon as a Function of Mass Number Nuclear Fission and Fusion Fusion: Combining two light nuclei to form a heavier, more stable nucleus. 3 1 4 0 2 He 1H 2 He 1e Fission: Splitting a heavy nucleus into two nuclei with smaller mass numbers. 1 235 142 91 1 0 n 92 U 56 Ba 36 Kr 30 n Figure 21.10 Both Fission and Fusion Produce More Stable Nuclides Figure 18.11 Fission Figure 18.12 Fission Produces a Chain Reaction Fission Processes A self-sustaining fission process is called a chain reaction. Neutrons Causing Event Fission subcritical <1 critical =1 supercritical > 1 Result reaction stops sustained reaction violent explosion Figure 18.13 Fission Produces Two Neutrons Key Parts of a Fission Reactor Because of tremendous energies involved, the fission process can be used as an energy source to produce electricity. Reactors were designed in which controlled fission can occur. The resulting energy is used to heat water to produce steam to run turbine generators. Reactor Core: 3% rods. Coolant Containment Shell 235 92 U + moderator and control Figure 18.14 A Schematic Diagram of a Nuclear Power Plant Figure 18.15 A Schematic Diagram of a Reactor Core Breeder Reactors Fissionable fuel is produced while the reactor runs ( 235 92 U is split, giving neutrons for the creation of 239 ): 94 Pu 1 0n 238 239 92 U 92 U 239 239 92 U 93 Np 239 239 93 Np 94 Pu 0 1 e 0 1e Biological Effects of Radiation • Somatic damage: Damage to the organism itself • Genetic damage: Damage to the genetic machinery. • Biological effects depend on: 1. Energy of the radiation 2. Penetration ability of the radiation 3. Ionizing ability of the radiation 4. Chemical properties of the radiation source