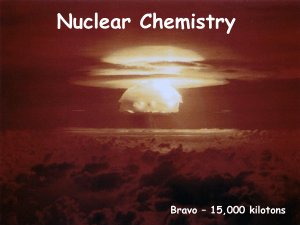
NUCLEAR CHEMISTRY
advertisement

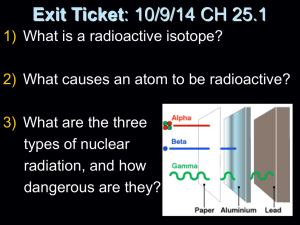
NUCLEAR CHEMISTRY Objectives 1 - 16 Objective 1 • Define radioactivity and distinguish between natural and artificial Objective 2 • Explain Transmutation Objective 3 • List three radioactive emissions: –Alpha –Beta –Gamma Objective 4 • Describe what each emission is composed of and how they differ from each other with respect to mass, charge, penetrating power, and ionizing power Objective 5 • Tell what happens to an element that undergoes alpha decay, beta decay, or gamma decay Objective 6 • Discuss the process used to separate the three types of radioactive emissions Separation of Particles Objective 7 • Discuss half life and use Table N to find half lives of various radioactive isotopes Objective 8 • Calculate half life and fraction remaining from various word problems Half life questions • How many grams of a 20 gram sample of iodine131 remain after 24.21 days? • What is the half life of a radioisotope if 2.5 grams of a 160 gram sample remains after 108 years? • If it takes 17190 years for a sample of a radioactive isotope to decay from 100 grams to 12.5 grams, what is the identity of the atom? Objective 9 • Solve for unknowns in nuclear equations Objective 10 • Define and explain mass defect Objective 11 • Define bonding energy Objective 12 • Explain the basic difference between a fission reaction and a fusion reaction Objective 13 • Explain how a chain reaction works Objective 14 • Discuss the difference between a fission reactor in a nuclear bomb and the one in a nuclear fission reactor Objective 15 • Give the details of a fusion reaction Objective 16 • List the three places fusion reactions occur