HW9.docx
advertisement
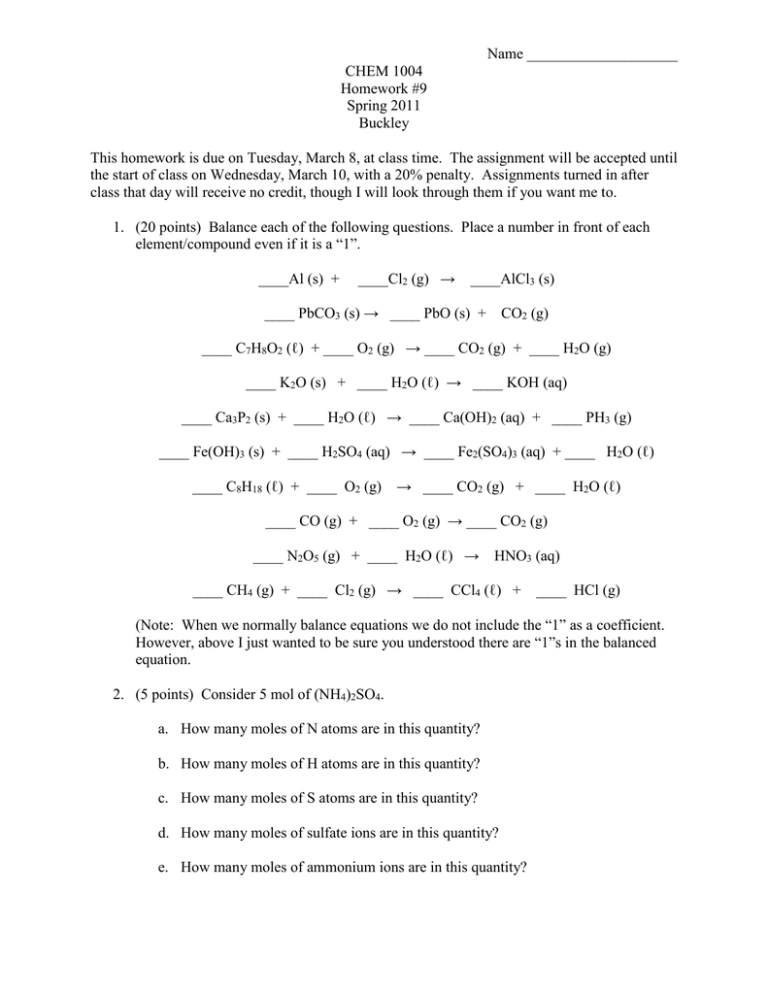
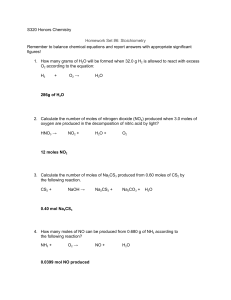
Name ____________________ CHEM 1004 Homework #9 Spring 2011 Buckley This homework is due on Tuesday, March 8, at class time. The assignment will be accepted until the start of class on Wednesday, March 10, with a 20% penalty. Assignments turned in after class that day will receive no credit, though I will look through them if you want me to. 1. (20 points) Balance each of the following questions. Place a number in front of each element/compound even if it is a “1”. ____Al (s) + ____Cl2 (g) → ____AlCl3 (s) ____ PbCO3 (s) → ____ PbO (s) + CO2 (g) ____ C7H8O2 (ℓ) + ____ O2 (g) → ____ CO2 (g) + ____ H2O (g) ____ K2O (s) + ____ H2O (ℓ) → ____ KOH (aq) ____ Ca3P2 (s) + ____ H2O (ℓ) → ____ Ca(OH)2 (aq) + ____ PH3 (g) ____ Fe(OH)3 (s) + ____ H2SO4 (aq) → ____ Fe2(SO4)3 (aq) + ____ H2O (ℓ) ____ C8H18 (ℓ) + ____ O2 (g) → ____ CO2 (g) + ____ H2O (ℓ) ____ CO (g) + ____ O2 (g) → ____ CO2 (g) ____ N2O5 (g) + ____ H2O (ℓ) → HNO3 (aq) ____ CH4 (g) + ____ Cl2 (g) → ____ CCl4 (ℓ) + ____ HCl (g) (Note: When we normally balance equations we do not include the “1” as a coefficient. However, above I just wanted to be sure you understood there are “1”s in the balanced equation. 2. (5 points) Consider 5 mol of (NH4)2SO4. a. How many moles of N atoms are in this quantity? b. How many moles of H atoms are in this quantity? c. How many moles of S atoms are in this quantity? d. How many moles of sulfate ions are in this quantity? e. How many moles of ammonium ions are in this quantity?